URINARY CATHETER, FOLEY 2 way, balloon, sterile, s.u., CH08
STD
SCTDCAUR08F
Valid Article
Account code:
60210
HS Code:
901839
Last Updated on:
02/05/2024, 22:21:38
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
U010201 - Urethral prostatic and bladder catheters, Nelaton, with balloon
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
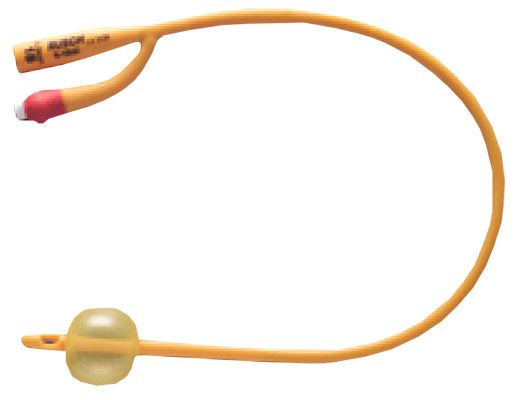
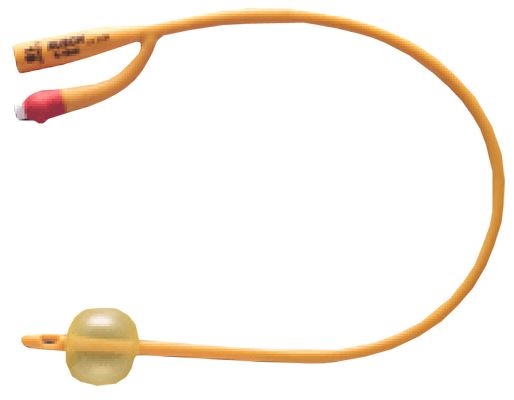
URINARY CATHETER, FOLEY
Definition
Foley urinary catheters are sterile, flexible tubes with an inflatable balloon at the distal tip intended to be inserted through the urethra and retained in the urinary bladder to function as an indwelling therapeutic device: they drain the bladder but block the urethra.
They have double lumens, or separate channels, running down it lengthwise = two-way catheters.
They are used in case of urinary incontinence (e.g., following a urological surgery procedure), or to void for non-ambulatory patients.
Synonym
Foley catheter, urethral catheter with balloon
Specifications
Quality standards
ISO 20696, 2018, edition 1, (confirmed 2023) Sterile urethral catheters for single use
Technical specifications
- Silicone coated latex
- The colour of the connecting piece or the filling opening of the balloon in Foley-type catheters represents a colour-code and designates the external diameter of the catheter.
| size | ext diam mm | colour |
| CH6 | 2.0 mm | light green |
| CH8 | 2.7 mm | blue |
| CH10 | 3.3 mm | black |
| CH12 | 4.0 mm | white |
| CH14 | 4.7 mm | green |
| CH16 | 5.3 mm | orange |
| CH18 | 6.0 mm | red |
| CH20 | 6.7 mm | yellow |
- Two-way catheter:
- central channel for urinary drainage:
- straight distal end with side eyes
- proximal end with funnel shape connector
- side channel for balloon inflation ending in a non-return valve with Luer connection
- Balloon capacity: the amount of sterile water to be inserted into the balloon is usually printed on the side of the nozzle end.
- 3 - 5 ml paediatric balloon
- 5 - 10 ml balloon for routine drainage in adults
- CH 6, CH 8, CH 10 size has a guide to facilitate catheterization
- Length: 30 to 45 cm
- Sterile, for single use
Packaging & Labelling
Double sterile packaging per unit in peel-open pack
Instructions for use
Please consult the “Manual of nursing Care Procedures, MSF, 2020” available online via the Nursing care working Group sharepoint page.
Some restricted information has been hidden. Sign in
to see this information
Precautions for Use
- Do not use in case of allergy to latex: use the silicone ones
- If clamping is required, clamp only the drainage channel. Clamping the whole catheter could obliterate the balloon channel causing difficulties with deflating the balloon.
- The balloon should be inflated with sterile water, never with air. Physiological saline (NaCl 0.9%) is not recommended as it may degrade the balloon.
- Do not inflate the balloon over the indicated maximum capacity.
- Never inflate the balloon without visualisation of urine drainage and advancing the catheter as this can cause urethral damage and pain.
- Consider a skin protection device and the type of tape to use for patients with a high-risk of developing a pressure ulcer or being injured by tape
Some restricted information has been hidden. Sign in
to see this information
Some restricted information has been hidden. Sign in
to see this information



![[KMEDMHOS45-] (mod OT Room) CATHETERS AND DRAINS 2021](/web/image/product.template/574392/image_256/%5BKMEDMHOS45-%5D%20%28mod%20OT%20Room%29%20CATHETERS%20AND%20DRAINS%202021?unique=4fbb9a8)
![[KMEDMHWS35-] (mod ward) CATHETERS, TUBES & DRAINS 2021](/web/image/product.template/574358/image_256/%5BKMEDMHWS35-%5D%20%28mod%20ward%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS%202021?unique=f41629f)
![[KMEDMHIS25-] (mod ICU) CATHETERS, TUBES and DRAINS 2021](/web/image/product.template/574344/image_256/%5BKMEDMHIS25-%5D%20%28mod%20ICU%29%20CATHETERS%2C%20TUBES%20and%20DRAINS%202021?unique=0478965)
![[KMEDMNUTI33] (module nut. inpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575289/image_256/%5BKMEDMNUTI33%5D%20%28module%20nut.%20inpatient%29%20MEDICAL%20SUPPLIES%202021?unique=0b15096)
![[KMEDMHES35-] (mod emergency) CATHETERS, TUBES & DRAINS 2021](/web/image/product.template/574355/image_256/%5BKMEDMHES35-%5D%20%28mod%20emergency%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS%202021?unique=3f6516f)
![[KMEDMHAS15-] (mod AMP) CATHETERS, TUBES & DRAINS](/web/image/product.template/572672/image_256/%5BKMEDMHAS15-%5D%20%28mod%20AMP%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS?unique=49d2a38)
![[KMEDMHCS15-] (mod OPD) CATHETERS, TUBES & DRAINS](/web/image/product.template/572722/image_256/%5BKMEDMHCS15-%5D%20%28mod%20OPD%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS?unique=49d2a38)