(API System) 20E STRIP, Enterobacteriaceae
Valid Article
API 20E STRIPS, Enterobacteriaceae
CAUTION
This test is worded and codified by unit to make the order easier, but it is always packaged in kit of 25 or 100 strips.
Definition
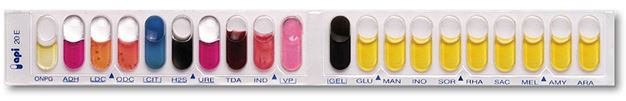
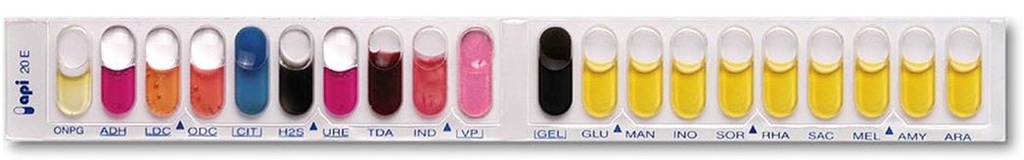
API 20 E is a standardized identification system for Enterobacteriaceae and other non-fastidious, Gram-negative rods which uses 20 miniaturized biochemical tests and a database.
Inoculation and reading are done manually, and identification is done with the software.
Specifications
Components
Kit of 25 tests (20100) containing:
- 25 API 20 E strips
- 25 incubation boxes
- 25 result sheets
- 1 clip seal
- 1 package insert
Kit of 100 tests (20160) containing:
- 100 API 20 E strips
- 100 incubation boxes
- 100 result sheets
- 1 clip seal
- 1 package insert
Technical specifications
Strip with 20 microtubes containing dehydrated substrates. Oxidase test should be done separately. The microtubes are inoculated with baterial suspension. The colours changes occur during incubation, due to metabolism, spontaneously or by addition of reagents.
Packaging & Labelling
Kit of 25 or 100 tests.
To be Ordered Separately
- API NaCl 0.85% medium, 5 ml
- Mineral oil
- API 20E reagent kit (NIT 1 + NIT 2, VP 1 + VP 2, TDA, JAMES)
- Zn reagent
- Oxidase
- Pipettes or PSIpettes
- Ampule rack and protector
- APIWEB software
Instructions for use
Please consult the “Bacteriology laboratory procedures and resources” available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources. https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Bacteriology-Laboratory-Resources.aspx
For offline access, contact your laboratory advisor
Precautions for Use
All biological samples must be considered as potentially infectious and handled with the usual precautions (compulsory wearing of gloves, hand washing, etc.).
Storage
- Store between 2°C and 8°C.
- Do not use after expiry date.
- Shelf life: 18 months.
- Guaranteed minimum remaining shelf life at delivery: 1/3 of the total shelf life.
- Once aluminium pouch is opened, the strips may be kept for up to 10 months at 2°C-8°C with desiccant sachets.
- Re-seal the pouch with the clip seal included in the kit.
Waste management
After use, sterilize the cultures and all contaminated material. Contact your watsan referent for proper disposal.
MSF requirements
Reserved to bacteriology programmes.



![[DINJSODC9A5] SODIUM chloride, 0.9%, 5ml, plastic amp.](/web/image/product.template/572829/image_256/%5BDINJSODC9A5%5D%20SODIUM%20chloride%2C%200.9%25%2C%205ml%2C%20plastic%20amp.?unique=f63d194)
![[ELABPIPT1S-] PIPETTE, TRANSFER, graduated, plastic, sterile, s.u.](/web/image/product.template/571075/image_256/%5BELABPIPT1S-%5D%20PIPETTE%2C%20TRANSFER%2C%20graduated%2C%20plastic%2C%20sterile%2C%20s.u.?unique=6706ba5)
![[ELABTUBE12-] TUBE, Ø 12 mm, plastic, sterile, 5 ml + PLUG](/web/image/product.template/571504/image_256/%5BELABTUBE12-%5D%20TUBE%2C%20%C3%98%2012%20mm%2C%20plastic%2C%20sterile%2C%205%20ml%20%2B%20PLUG?unique=5359831)
![[ELAEDISE3--] DISTILLER (Tuttnauer EWS-RD1), 0.95L/h, 3.8L, 220V, 50/60Hz](/web/image/product.template/572599/image_256/%5BELAEDISE3--%5D%20DISTILLER%20%28Tuttnauer%20EWS-RD1%29%2C%200.95L-h%2C%203.8L%2C%20220V%2C%2050-60Hz?unique=a6b1e9a)
![[SBIDAPISJAMES] (API System) JAMES reagent (NH,10S,20E,20NE),kit [BMX-70542]](/web/image/product.template/572905/image_256/%5BSBIDAPISJAMES%5D%20%28API%20System%29%20JAMES%20reagent%20%28NH%2C10S%2C20E%2C20NE%29%2Ckit%20%5BBMX-70542%5D?unique=ebf3b6d)
![[SBIDAPISNIT] (APISyst) NIT1-NIT2 reagents (10S,20E,20NE,STAPH)[BMX-70442]](/web/image/product.template/572908/image_256/%5BSBIDAPISNIT%5D%20%28APISyst%29%20NIT1-NIT2%20reagents%20%2810S%2C20E%2C20NE%2CSTAPH%29%5BBMX-70442%5D?unique=387ad3e)
![[SBIDAPISOIL] (API System) PARAFFIN OIL, 125 ml [BMX-70100]](/web/image/product.template/572927/image_256/%5BSBIDAPISOIL%5D%20%28API%20System%29%20PARAFFIN%20OIL%2C%20125%20ml%20%5BBMX-70100%5D?unique=d9319e6)
![[SBIDAPISTDA] (API System) TDA reagent (10S, 20E), kit [BMX-70402]](/web/image/product.template/572904/image_256/%5BSBIDAPISTDA%5D%20%28API%20System%29%20TDA%20reagent%20%2810S%2C%2020E%29%2C%20kit%20%5BBMX-70402%5D?unique=4f4d980)
![[SBIDAPISVP-] (API System) VP1-VP2 reagents (20E,STREP,STAPH) [BMX-70422]](/web/image/product.template/572903/image_256/%5BSBIDAPISVP-%5D%20%28API%20System%29%20VP1-VP2%20reagents%20%2820E%2CSTREP%2CSTAPH%29%20%5BBMX-70422%5D?unique=387ad3e)
![[SBIDAPISZN-] (API System) ZN reagent (20E, 20NE), kit [BMX-70380]](/web/image/product.template/572912/image_256/%5BSBIDAPISZN-%5D%20%28API%20System%29%20ZN%20reagent%20%2820E%2C%2020NE%29%2C%20kit%20%5BBMX-70380%5D?unique=4fe1b84)