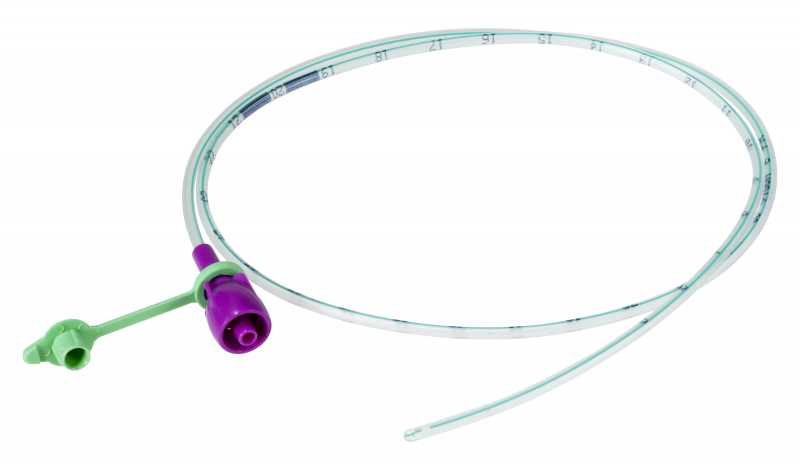
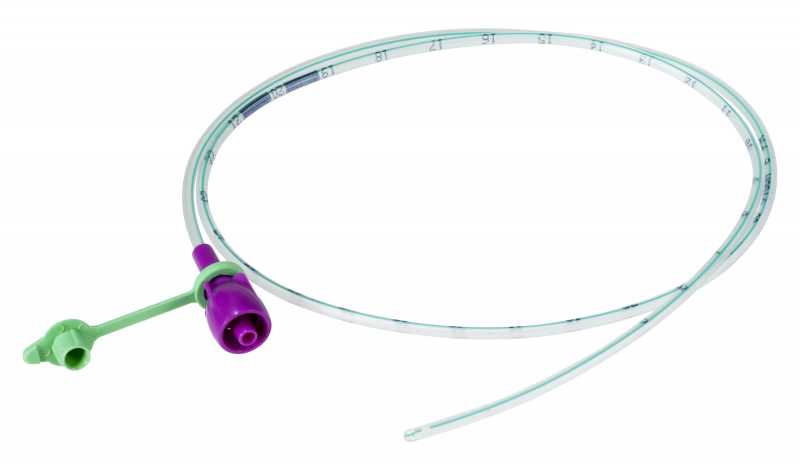
NASOGASTRIC TUBE, ENFit tip, s.u., 50 cm, CH06, light green
STD
SCTDTUGF06-
Valid Article
Account code:
60210
HS Code:
901890
Last Updated on:
02/05/2024, 23:05:54
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
G02020101 - Nasogastric tubes
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
NASOGASTRIC TUBE, ENFit tip
Definition
A sterile, thin, flexible, hollow cylinder intended to access the stomach of a patient through the nose and nasopharynx primarily for enteral feeding and the administration of medications, either by gravity or with a pump. It is typically a single-lumen plastic tube of various diameters (typically smaller than those of nasogastric tubes intended primarily for decompression) with circular markings that serve as insertion guides.
The NG-tube is designed with a specific ENFit end so that you can only use products designed for enteral/tube feeding access.
Specifications
Quality standards
ISO 80369-3, 2016, edition 1, +A1 2019 Small-bore connectors for liquids and gases in healthcare applications - Part 3: Connectors for enteral applications
Quality Standards Comment
See information on nasogastric and enteral tubes
Technical specifications
- Transparent
- PVC or polyurethane
- Single channel
- Closed distal end, with minimum 2 oval lateral eyes with atraumatic edges
- Proximal end with purple ENFit connection + stopper
- Radio-opaque or with radio-opaque markings for easy positioning (minimum / 5 cm)
- Possibility of identification of the size once the tube is out of the packaging, by colour code is preferred(coloured ring built into the connector, coloured closure cap, coloured attachement of the closure cap)
- Sterile, for single use
- Dimensions:
- Length: 40 to 60 cm (CH6 - 10) or 90 cm (CH12 & 14)
- Diameter: CH6, CH8, CH10, CH 12 and CH 14
- Size indicated by an international colour code:
| size | ext diam mm | colour |
| CH6 | 2.0 mm | light green |
| CH8 | 2.7 mm | blue |
| CH10 | 3.3 mm | black |
| CH12 | 4.0 mm | white |
| CH14 | 4.7 mm | green |
Packaging & Labelling
Unit sterile packaging in peel-open pack
Instructions for use
Some restricted information has been hidden. Sign in
to see this information
Precautions for Use
- Confirm the correct position of nasogastric tubes
- Prevent misconnection errors
See information on nasogastric and enteral tubes
Some restricted information has been hidden. Sign in
to see this information



![[KMEDMHWS35-] (mod ward) CATHETERS, TUBES & DRAINS 2021](/web/image/product.template/574358/image_256/%5BKMEDMHWS35-%5D%20%28mod%20ward%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS%202021?unique=f41629f)
![[KMEDMHIS25-] (mod ICU) CATHETERS, TUBES and DRAINS 2021](/web/image/product.template/574344/image_256/%5BKMEDMHIS25-%5D%20%28mod%20ICU%29%20CATHETERS%2C%20TUBES%20and%20DRAINS%202021?unique=0478965)
![[KMEDMNUTI33] (module nut. inpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575289/image_256/%5BKMEDMNUTI33%5D%20%28module%20nut.%20inpatient%29%20MEDICAL%20SUPPLIES%202021?unique=0b15096)
![[KMEDMSUP04S1] (supplementary unit 2018) MODULE RENEWABLE SUPPLIES 2019](/web/image/product.template/569226/image_256/%5BKMEDMSUP04S1%5D%20%28supplementary%20unit%202018%29%20MODULE%20RENEWABLE%20SUPPLIES%202019?unique=4b6099e)
![[KMEDMHIE231] (mod ICU) SPECIALIZED NUTRITION 2021](/web/image/product.template/574341/image_256/%5BKMEDMHIE231%5D%20%28mod%20ICU%29%20SPECIALIZED%20NUTRITION%202021?unique=6fb9a30)
![[KMEDMCHO021] (module 001) RENEWABLE SUPPLIES 2019](/web/image/product.template/569222/image_256/%5BKMEDMCHO021%5D%20%28module%20001%29%20RENEWABLE%20SUPPLIES%202019?unique=9e0e0a7)
![[KMEDMHDS21-] (mod delivery & neonate) RENEWABLE SUPPLIES compl. 2019](/web/image/product.template/569223/image_256/%5BKMEDMHDS21-%5D%20%28mod%20delivery%20%26%20neonate%29%20RENEWABLE%20SUPPLIES%20compl.%202019?unique=f928ae9)
![[KMEDMHCS211] (mod OPD) COMPLEMENTARY RENEWABLE SUPPLIES 2021](/web/image/product.template/574350/image_256/%5BKMEDMHCS211%5D%20%28mod%20OPD%29%20COMPLEMENTARY%20RENEWABLE%20SUPPLIES%202021?unique=d19c638)
![[EEMDENPC102] (pump Flocare) ADMINISTRATION SET, ENFit, ster.,s.u. 586514](/web/image/product.template/573221/image_256/%5BEEMDENPC102%5D%20%28pump%20Flocare%29%20ADMINISTRATION%20SET%2C%20ENFit%2C%20ster.%2Cs.u.%20586514?unique=a6e7037)
![[EEMDENPE1--] ENTERAL NUTRITION PUMP (Flocare Infinity) 230V with battery](/web/image/product.template/570854/image_256/%5BEEMDENPE1--%5D%20ENTERAL%20NUTRITION%20PUMP%20%28Flocare%20Infinity%29%20230V%20with%20battery?unique=e11cd1e)
![[ELABPAPEPH2G] PAPER, pH INDICATOR, 2 to 9, graduation 0.5, gastric, strip](/web/image/product.template/569029/image_256/%5BELABPAPEPH2G%5D%20PAPER%2C%20pH%20INDICATOR%2C%202%20to%209%2C%20graduation%200.5%2C%20gastric%2C%20strip?unique=921eba6)
![[SCTDAENG1--] ADMINISTRATION SET EN, gravity, ENPlus/ENFit, ster.,s.u.](/web/image/product.template/571924/image_256/%5BSCTDAENG1--%5D%20ADMINISTRATION%20SET%20EN%2C%20gravity%2C%20ENPlus-ENFit%2C%20ster.%2Cs.u.?unique=937acd4)
![[SCTDTUGH1A-] FIXATION PLASTER for NASOGASTRIC TUBE, s.u., adult / child](/web/image/product.template/569568/image_256/%5BSCTDTUGH1A-%5D%20FIXATION%20PLASTER%20for%20NASOGASTRIC%20TUBE%2C%20s.u.%2C%20adult%20-%20child?unique=62e6069)
![[SCTDTUGH1N-] FIXATION PLASTER for NASOGASTRIC TUBE, s.u., neonate](/web/image/product.template/569567/image_256/%5BSCTDTUGH1N-%5D%20FIXATION%20PLASTER%20for%20NASOGASTRIC%20TUBE%2C%20s.u.%2C%20neonate?unique=62e6069)
![[SDRETAPA1F25] ADHESIVE TAPE, elastic foam, 2-2.5cm x 5m](/web/image/product.template/569593/image_256/%5BSDRETAPA1F25%5D%20ADHESIVE%20TAPE%2C%20elastic%20foam%2C%202-2.5cm%20x%205m?unique=24a6bf6)