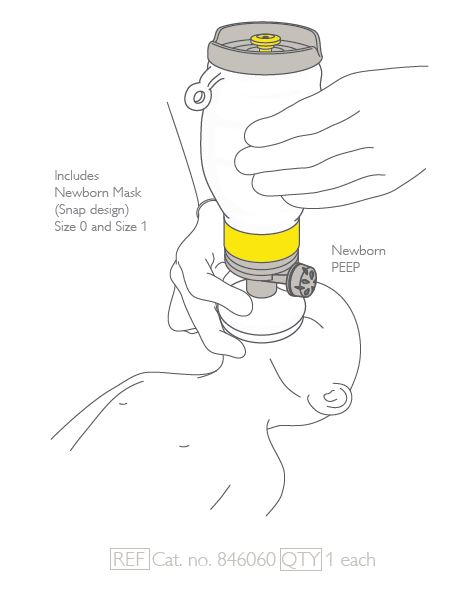
RESUSCITATOR (Laerdal Upright), neonate, PEEP + MASK n°0&1
Valid Article
RESUSCITATOR, neonate, upright (Laerdal)
Definition
A hand-operated device designed to provide or assist ventilation in newborns who are apnoeic or exhibit inadequate respiration. It employs ambient air and includes a large flexible chamber that is hand-ventilated, a gas reservoir (oxygen reservoir), tubing, and a connector for attachment to a mask or endotracheal tube; oxygen from an O2 source may also be connected when necessary.
Specifications
Two different models:
- Basic upright resuscitator model
- Model with the positive end expiratory pressure (PEEP) to the upright resuscitator prevents repeated alveolar collapse during ventilation, helps to recruit lung volume more efficiently, improves the clearance of lung fluid at birth and reduces damage to lung tissue.
Quality standards
Technical specifications
Resuscitator EANERESU1NU and EANERESU1NUP
- SELF-INFLATING BALLOON
- made of transparent silicone
- maximum tidal volume: 320 ml
- maximum stroke volume: 150 ml
- patient valve connection
- INLET VALVE FOR AMBIENT AIR
- PATIENT VALVE
- non rebreathing device, one-way, neonate model
- with pressure relieve valve
- translucent polysulphone
- female respiratory connector ISO 24 mm
- MASK nº S1 and S0
- transparent silicone
- adjustable on 22/15 mm connections
- PEEP valve for EANERESU1NUP
- principle : silicone membrane valve
- reusable
- is screwed on the distal end
- pressure range : 6 ± 2 cm H2O
- Easy to disassemble for cleaning
- All components are autoclavable up to 134ºC
- Non sterile
To be Ordered Separately
- Oxygen kit complete: EANERESU1NU1: reservoir made of transparent plastic, with silicone tubing, with valve connection (which is different compared to the previous/old horizontal (elbow) resuscitator)
- Additional silicone masks size 0 and 1: minimum quantity in stock = 10 pieces of each size!
Instructions for use
Forms part of the emergency resuscitation equipment. Should always be kept ready for use: visible, clean, complete and in good working order.
Precautions for Use
Filters for breathing circuit to be used between the mask and the balloon to minimize the risk of cross contamination. The filter must be changed between each patient
Maintenance
Before use
- Check that the valve outlet operates correctly (it should not stick), by gently breathing through the valve whilst watching the flap move.
- Check the elasticity of the bag by manual pressure.
After each use
- The patient valve and the masks must be pre-disinfected, cleaned and sterilized by steam autoclave
(Cf Introduction: Disinfection and sterilization in the field)
MSF requirements
Device used for manual ventilation assistance in neonates and babies with weight is <10 kg in case of respiratory distress or failure. Oxygen Reservoir Bag with included valve and tubing allows to administer 100% oxygen to the new-born.
The upright bag and mask has been designed to improve the efficacy of ventilation in newborns.