SYPHILIS RPR TEST, carbon test, ser/pl, 1test (ARL. 900025)
Valid Article
SYPHILIS RPR TEST (Arlington)
CAUTION
This test is worded and codified by unit to make the order easier, but it is always packaged in kit of 25 or 100.
Definition
Qualitative or semi-quantitative non-treponema agglutination test on card using a VDRL (Veneral Disease Research Laboratory) antigen combined with carbon particles.
Used for the screening and the follow-up of syphilis in human serum or plasma.
Remark
Compared with the RPR test, the syphilis RDT (see related articles below) has two practical advantages:
- it can be performed on whole blood, serum or plasma
- it does not require cold chain
but it doesn't allow the follow-up after treatment
Synonym
RPR = Rapid Plasma Reagin
Specifications
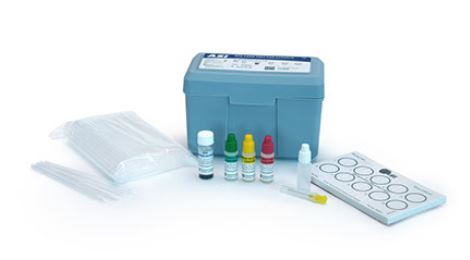
Components
- RPR Test cards (10-well) (3 or 10)
- RPR Carbon antigen (0.5ml or 2.0ml)
- Reactive control (0.5ml or 2.0ml)
- Weak reactive control (0.5ml or 2.0ml)
- Non reactive control (0.5ml or 2.0ml)
- 3 ml Dropping bottle and 20G needle
- Disposable stirrer pipets (25 or 100)
Technical specifications
- Sample type: serum or plasma (EDTA)
- Sample volume: at least 0,5 ml
Packaging & Labelling
Kit of 25 or 100 tests
Transport Dangerous Goods
Transport not regulated
Instructions for use
Follow the instructions.
Please consult the “Updated laboratory procedures, 2022” available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources.
https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Laboratory-Manual-page.aspx
For offline access, contact your laboratory advisor.
Precautions for Use
All biological samples must be considered as potentially infectious and handled with the usual precautions (compulsary wearing of gloves, hand washing, etc.).
The reagent may contain dangerous products (see the leaflet of the manufacturer).
Storage
- Keep refrigerated between 2 and 8ºC
- Do not freeze
- Shelf life: 18 mois
- Guaranteed minimum remaining shelf life at delivery: 1/3 of total shelf life
- Not to be used beyond expiry date
Waste management
Incinerate the cards and the used materials.
Classification EC Regulation N° 1272/2008
Not classified as hazardous
MSF requirements
Inexpensive.
Quantitative and qualitative methods are possible.



![[ELAEROTE2--] ROTATOR, orbital, for agglutination test, 230 V](/web/image/product.template/572455/image_256/%5BELAEROTE2--%5D%20ROTATOR%2C%20orbital%2C%20for%20agglutination%20test%2C%20230%20V?unique=393df5d)
![[SSDTSYPT25T3] SYPHILIS TEST, ser/pl/wb, 1 test (First response PI08FRC25)](/web/image/product.template/574437/image_256/%5BSSDTSYPT25T3%5D%20SYPHILIS%20TEST%2C%20ser-pl-wb%2C%201%20test%20%28First%20response%20PI08FRC25%29?unique=aa8ba5c)
![[SSDTSYPT30T] SYPHILIS TEST, ser/pl/wb,1 test(Bioline Syphilis 3.0 06FK10)](/web/image/product.template/569162/image_256/%5BSSDTSYPT30T%5D%20SYPHILIS%20TEST%2C%20ser-pl-wb%2C1%20test%28Bioline%20Syphilis%203.0%2006FK10%29?unique=7b479b6)