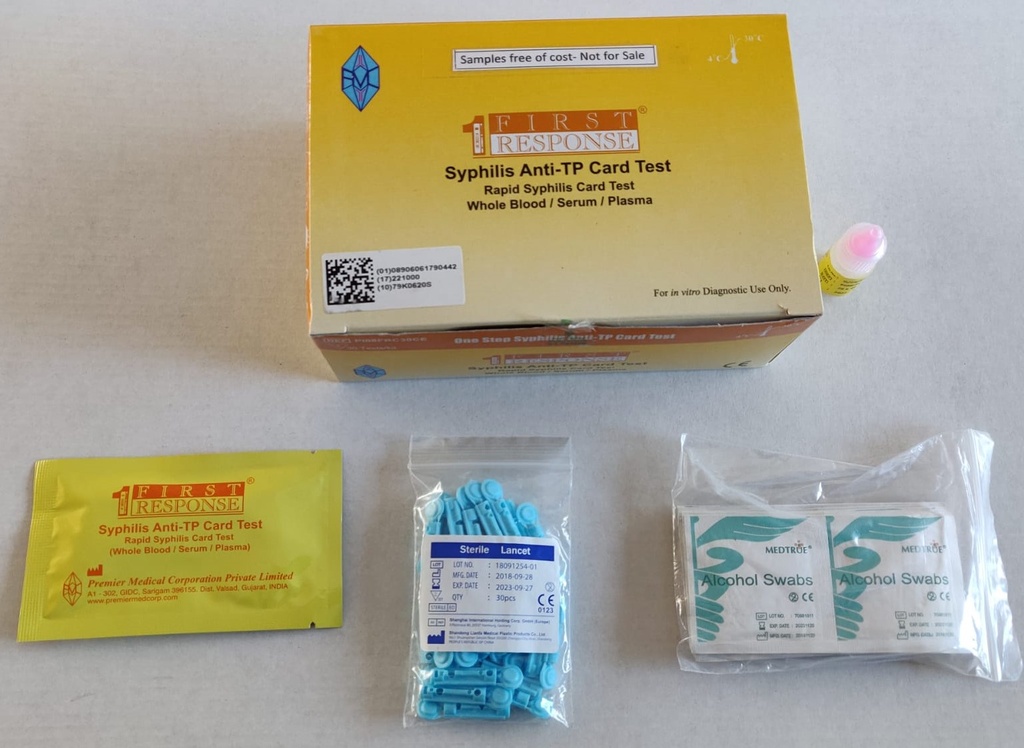
SYPHILIS TEST, ser/pl/wb, 1 test (First response PI08FRC25)
Valid Article
SYPHILIS TEST (FIRST RESPONSE)
CAUTION
This test is worded and codified by unit to make the order easier, but it is always packaged in kit of 25.
Definition
Rapid immunochromatographic test for the qualitative detection of anti-treponemal antibodies.
Used for the screening of syphilis in blood donors and pregnant women.
Remark
The syphilis RDT has two practical advantages compared with the RPR syphilis test:
- it can be performed on whole blood, serum or plasma
- it does not require cold chain
but it doesn't allow the follow-up after treatment
Specifications
Quality Standards Comment
Included in the WHO Model List of Essential In Vitro Diagnostics 2019
Components
- 25 x test devices with desiccant in foil pouch
- 25 x specimen transfer device
- 1 assay buffer bottle (1x 2.5ml)
- 25 x lancets
- 25 x alcohol swabs
- 1 instructions for use
Technical specifications
- Single device
- Two-step rapid test (20-25 minutes)
- Sample type: serum/plasma/whole blood (venous or capillary)
- Sample volume: 20µl
- According to the WHO PQ report, January 2021:
- Sensitivity on serum/plasma: 99.6% (98.0-100)
- Specificity on serum/plasma: 94.9% (98.4-100)
Packaging & Labelling
Box of 25 tests
Instructions for use
Follow the instructions in the leaflet.
Can be performed on serum, plasma or whole blood.
Within the MSF pretransfusional protocol, it is recommended to collect a single blood sample in a tube with anticoagulant, EDTA if possible, in order to perform all the necessary tests (blood group determination, screening for hepatitis, HIV, syphilis and malaria).
Please consult the “Updated laboratory procedures, 2022” available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources.
https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Laboratory-Manual-page.aspx
For offline access, contact your laboratory advisor.
- See: Blood transfusion, MSF, 2019
- See: Essential Obstetric and New-born care, MSF, 2019
- See: Updated laboratory procedures, MSF, 2022
- See: WHO Model List of Essential In Vitro Diagnostics 2023
- See: Syphilis test First Response SSDTSYPT25T3 SOP, MSF, 2023
- See: Syphilis test First Response SSDTSYPT25T3 BA, MSF, 2022
- See: MSF diagnostic packages, MSF, 2023
Precautions for Use
All biological samples must be considered as potentially infectious and handled with the usual precautions (compulsory wearing of gloves, hand washing, etc.).
Storage
- Store between 2 - 30º C
- Do not freeze
- Shelf life: 24 months
- Not to be used beyond expiry date
Waste management
Incinerate the tests and the used materials.
MSF requirements
Two - step rapid test.
No need for cold chain.




![[ELABPIAA0100] PIPETTE, AUTOMATIC, adjustable vol. 10-100 µl (Eppendorf)](/web/image/product.template/570956/image_256/%5BELABPIAA0100%5D%20PIPETTE%2C%20AUTOMATIC%2C%20adjustable%20vol.%2010-100%20%C2%B5l%20%28Eppendorf%29?unique=4283584)
![[ELABPIATY--] (aut.pip.) TIP YELLOW, 2-200µl (Eppdf)](/web/image/product.template/571110/image_256/%5BELABPIATY--%5D%20%28aut.pip.%29%20TIP%20YELLOW%2C%202-200%C2%B5l%20%28Eppdf%29?unique=0a70ae5)
![[ELABPIDF20-] PIPETTE, fixed volume (MiniPet), 20 µl pink](/web/image/product.template/571124/image_256/%5BELABPIDF20-%5D%20PIPETTE%2C%20fixed%20volume%20%28MiniPet%29%2C%2020%20%C2%B5l%20pink?unique=9e0a09d)
![[SSDTSYPT100T2] SYPHILIS RPR TEST, carbon test, ser/pl, 1test (ARL. 900100)](/web/image/product.template/572288/image_256/%5BSSDTSYPT100T2%5D%20SYPHILIS%20RPR%20TEST%2C%20carbon%20test%2C%20ser-pl%2C%201test%20%28ARL.%20900100%29?unique=5146fca)
![[SSDTSYPT25T] SYPHILIS RPR TEST, carbon test, ser/pl, 1test (ARL. 900025)](/web/image/product.template/568861/image_256/%5BSSDTSYPT25T%5D%20SYPHILIS%20RPR%20TEST%2C%20carbon%20test%2C%20ser-pl%2C%201test%20%28ARL.%20900025%29?unique=fa61836)
![[STSSLANCSAM2] SAFETY LANCET, medium flow, needle 21G x 1.8mm, green, s.u.](/web/image/product.template/569100/image_256/%5BSTSSLANCSAM2%5D%20SAFETY%20LANCET%2C%20medium%20flow%2C%20needle%2021G%20x%201.8mm%2C%20green%2C%20s.u.?unique=4e619c7)
![[SSDTSYPT30T] SYPHILIS TEST, ser/pl/wb,1 test(Bioline Syphilis 3.0 06FK10)](/web/image/product.template/569162/image_256/%5BSSDTSYPT30T%5D%20SYPHILIS%20TEST%2C%20ser-pl-wb%2C1%20test%28Bioline%20Syphilis%203.0%2006FK10%29?unique=7b479b6)