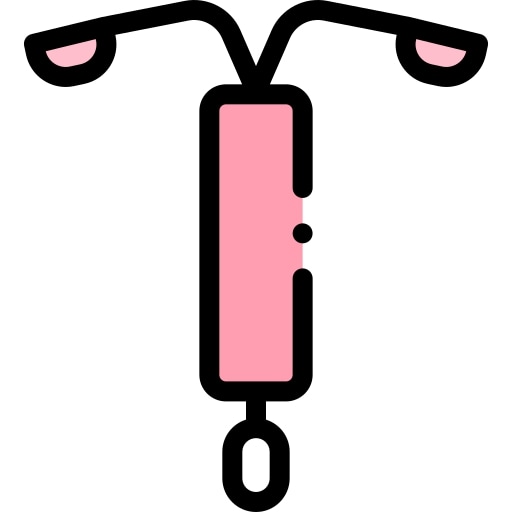
INTRA UTERINE DEVICE, LEVONORGESTREL, 52mg (LNG-IUD 52)
Valid Article
INTRA UTERINE DEVICE, LEVONORGESTREL
Full Name
Synonym: hormonal IUD
Therapeutic Action
T‐shaped IUD polyethylene device measuring 32 mm long and 32 mm wide, with a steroid reservoir containing 52 mg of levonorgestrel released at an initial rate of 20 μg daily.
Indications
Long-acting contraception, for a maximum of 6 years (Abivela®) or 5 years (Mirena®), after which the device must be changed.
Treatment of heavy bleeding during monthly periods (menstruation), fibroids and endometriosis, etc.
Protection from endometrial hyperplasia during oestrogen replacement therapy
Instructions for use
For insertion, provide the I.U.D. FITTING SET, 7 instruments.
For the conditions of insertion and removal, follow the manufacturer's instructions.
Remarks:
Mirena® and Abivela® brands are equivalent.
Abivela® marqueted by Medicines 360, is less costly but distribution rights are restricted to 88 countries.
When ordering, check with your supply centre for the brand registered and allowed in the mission/country concerned.
Precautions for Use
Contraindications: breast cancer, cervical cancer, genital tract infection, uterine abnormality that prevents correct IUD placement, severe liver disease (cirrhosis) or liver tumours, pelvic tuberculosis.
The hormonal IUD can be inserted immediately after delivery and can be safely used during breastfeeding.
Storage
Below 25ºC - Protect from sunlight


![[KSURBIUD7--] I.U.D. FITTING SET, 7 instruments](/web/image/product.template/570400/image_256/%5BKSURBIUD7--%5D%20I.U.D.%20FITTING%20SET%2C%207%20instruments?unique=e6e353d)
![[SMSUIUDE1C-] INTRA UTERINE DEVICE, (I.U.D.), copper, TCU 380 A](/web/image/product.template/568705/image_256/%5BSMSUIUDE1C-%5D%20INTRA%20UTERINE%20DEVICE%2C%20%28I.U.D.%29%2C%20copper%2C%20TCU%20380%20A?unique=a2e7517)