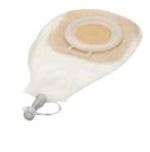
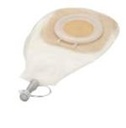
DRAINAGE POUCH, Ø 70-80 mm, >= 450 ml, window, ster. s.u.
Valid Article
WOUNDPOUCH, drainable
It is possible that not all sizes are available at all times, please contact your supply centre for exact information about availability.
Definition
A sterile plastic pouch designed to be attached to the skin around a large wound or fistula and intended to be used as a receptacle for exudate, blood, or other bodily wastes. It is a one-piece device, with a large-area adhesive flange that can be cut to fit closely around a wound, and which provides some skin protection. It has a tubular outlet to be connected to a drain system.
Specifications
Quality standards
Technical specifications
One-piece sterile, single use device with adhesive base plate (wafer) and collection pouch (similar to a large one-piece stoma bag)
Can remain in place up to 7 days.
BASE PLATE
- Pliable flat base plate with adhesive properties
- Opening should be able to be cut to various shapes (cut-to-fit)
- Composition of hydrocolloid material that protects the skin and wound edges from corrosive fistula output
POUCH
- Transparent pouch with at least one dependent drainage opening that can be closed-off fluid-tight and allows connection via conical connection piece to a drainage bag (e.g. standard urine bag)
- With hinged access window to allow for inspection or dressing of the wound
- Capacity: minimum 450 ml ( SCTDBAWD1--and SCTDBAWD3--) or 1900 ml (SCTDBAWD2--)
To be Ordered Separately
Peristomal dressing rings are quite effective in making irregular perifistular surfaces, smoother, hence facilitating adhesion of the pouches; they also allow irritated surrounding skin to heal as they work as a barrier seal between the edge of the fistula/wound and the bag wafer. They can be moulded and reshaped to fit irregular and odd-shaped lesions.
MSF requirements
The large pouch can be used for the treatment of open abdomen while the small one is used for fistula care.



![[SCTDBACO3D-] (intestinal ostomy bag) PERISTOMAL DRESSING, paste 60 g tube](/web/image/product.template/571680/image_256/%5BSCTDBACO3D-%5D%20%28intestinal%20ostomy%20bag%29%20PERISTOMAL%20DRESSING%2C%20paste%2060%20g%20tube?unique=0a028ad)
![[SCTDBACODR1-] PERISTOMAL DRESSING RING, 98 x 2.3mm (16.5g)](/web/image/product.template/572718/image_256/%5BSCTDBACODR1-%5D%20PERISTOMAL%20DRESSING%20RING%2C%2098%20x%202.3mm%20%2816.5g%29?unique=8722e01)
![[SCTDBAGU2VSS] URINE BAG 2 l, drain., non-ret. valves, sampling port, ster.](/web/image/product.template/571478/image_256/%5BSCTDBAGU2VSS%5D%20URINE%20BAG%202%20l%2C%20drain.%2C%20non-ret.%20valves%2C%20sampling%20port%2C%20ster.?unique=8722e01)