TEST HIV1+2/SYPHILIS, ser/pl/wb, 1test (First Resp.I20FRC25)
Valid Article
HIV1+2/SYPHILIS TEST (First Response)
CAUTION
This test is worded and codified by unit to make the order easier, but it is always packaged in kit of 25
Definition
A qualitative screening in vitro diagnostic test for detection of antibodies of HIV type 1 & 2 (IgM and IgG) and T. pallidum in human whole blood, serum or plasma and capillary blood.
Specifications
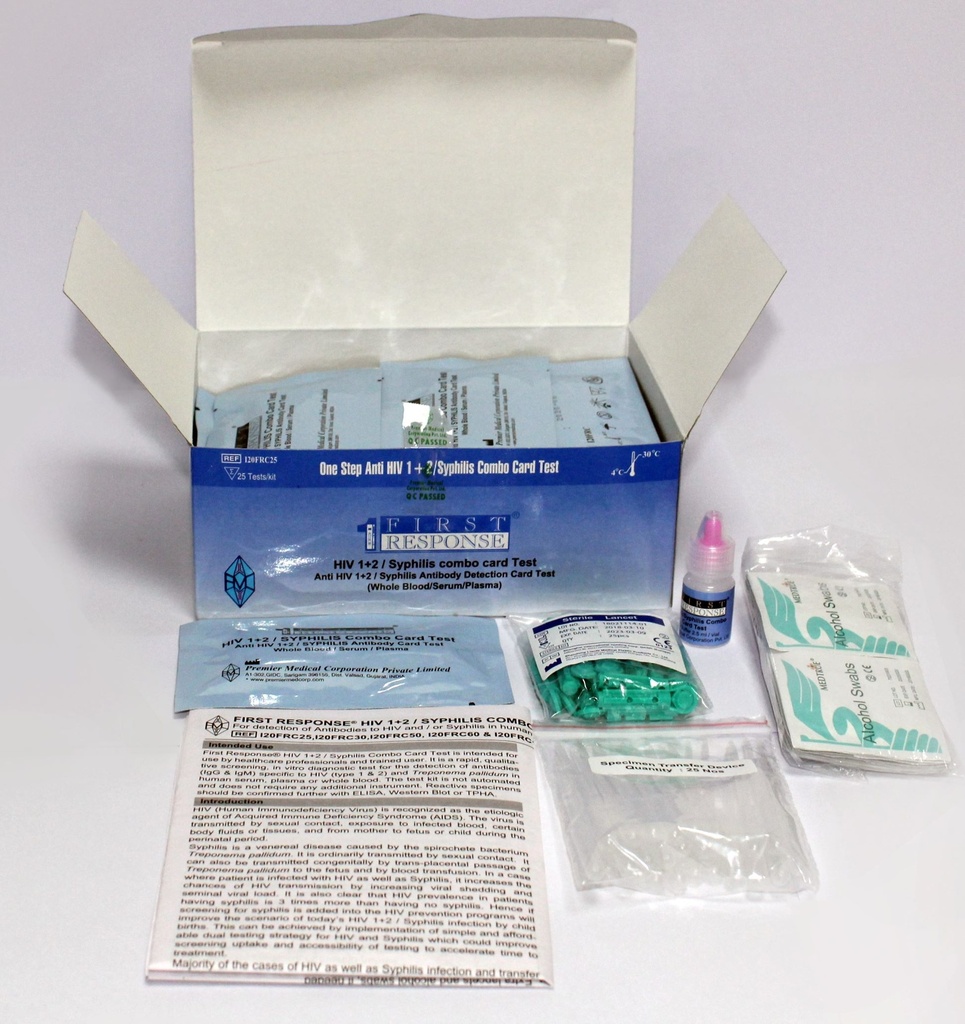
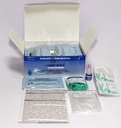
Components
- 25 x test devices with desiccant in individual foil pouches
- 25 x specimen transfer device
- 1 x assay diluent (2.5 ml vial)
- 25 x lancets
- 25 x alcohol swabs
- 1 instructions for use
Technical specifications
- Sample type: whole blood, serum, plasma
- Technology: rapid immunochromatographic assay
- Format: lateral flow cassette, 25 tests
- Sample type: serum, plasma, whole blood (venous or capillary)
- Sample volume: 20µl
- Time to result: 15-25 minutes
- Performance according to WHO PQ report (February 2020);
Instructions for use
Follow the instructions in the leaflet.
Please consult the “Updated laboratory procedures, 2022” available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources.
https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Laboratory-Manual-page.aspx
For offline access, contact your laboratory advisor.
Storage
- Between 4 - 30°C, Protect from sunlight - Protect from humidity
- Do not freeze reagents
- Shelflife: 24 months
Waste management
Incinerate the tests and the used materials.
MSF requirements
For specific programs only, please refer to your laboratory advisor.


![[ELABPIAA0100] PIPETTE, AUTOMATIC, adjustable vol. 10-100 µl (Eppendorf)](/web/image/product.template/570956/image_256/%5BELABPIAA0100%5D%20PIPETTE%2C%20AUTOMATIC%2C%20adjustable%20vol.%2010-100%20%C2%B5l%20%28Eppendorf%29?unique=ba1485a)
![[ELABPIATY--] (aut.pip.) TIP YELLOW, 2-200µl (Eppdf)](/web/image/product.template/571110/image_256/%5BELABPIATY--%5D%20%28aut.pip.%29%20TIP%20YELLOW%2C%202-200%C2%B5l%20%28Eppdf%29?unique=094948a)
![[ELABTIME1E-] TIMER, electronic](/web/image/product.template/571049/image_256/%5BELABTIME1E-%5D%20TIMER%2C%20electronic?unique=a8ec56b)
![[STSSBSVT5E-] (blds.syst.) TUBE, VACUUM, plastic, K2EDTA, 4ml, purple](/web/image/product.template/570499/image_256/%5BSTSSBSVT5E-%5D%20%28blds.syst.%29%20TUBE%2C%20VACUUM%2C%20plastic%2C%20K2EDTA%2C%204ml%2C%20purple?unique=aadad37)
![[STSSLANCSHL1] SAFETY LANCET, low flow, needle 28Gx1.6mm, light blue, s.u.](/web/image/product.template/570578/image_256/%5BSTSSLANCSHL1%5D%20SAFETY%20LANCET%2C%20low%20flow%2C%20needle%2028Gx1.6mm%2C%20light%20blue%2C%20s.u.?unique=5ab9f2d)
![[SSDTHISY25T3] TEST HIV1+2/SYPHILIS, ser/pl/wb, 1test (Standard Q 09HIV20D)](/web/image/product.template/577877/image_256/%5BSSDTHISY25T3%5D%20TEST%20HIV1%2B2-SYPHILIS%2C%20ser-pl-wb%2C%201test%20%28Standard%20Q%2009HIV20D%29?unique=28a5dd2)