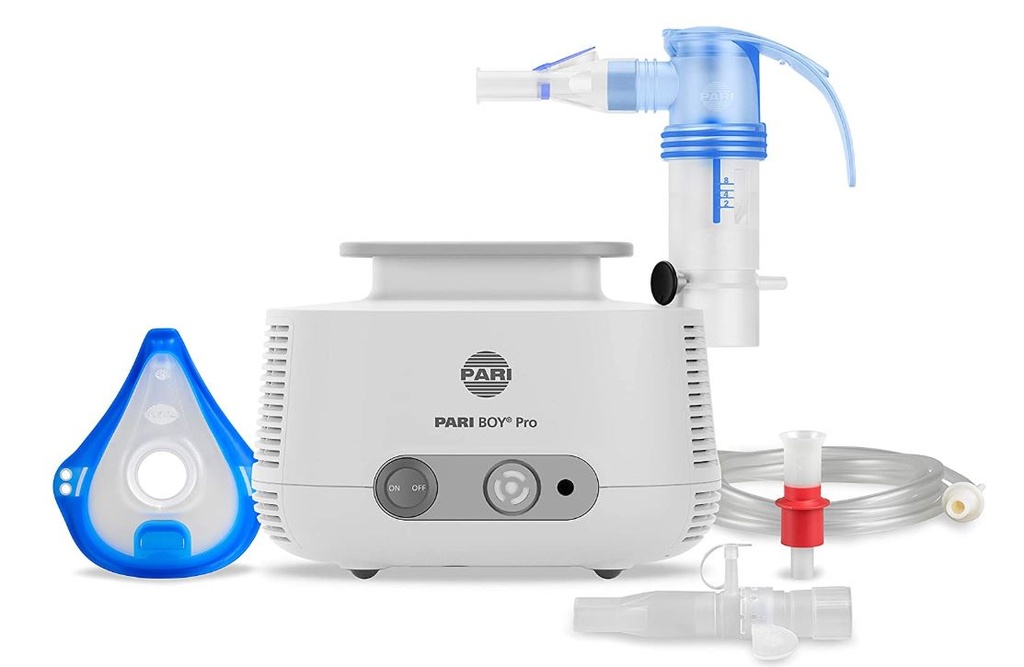
NEBULIZER + COMPRESSOR (PARI BOY PRO) + accessories
Valid Article
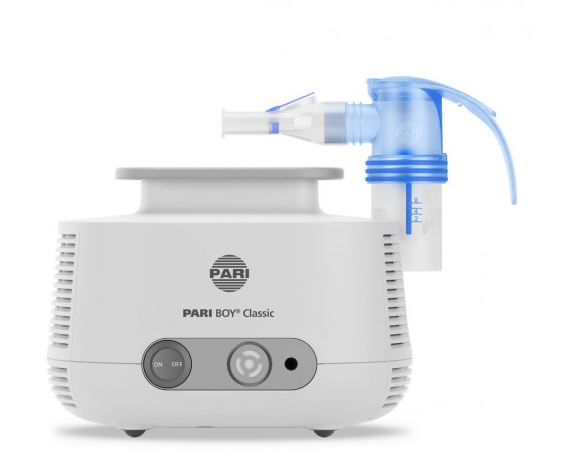
NEBULIZER + COMPRESSOR (PARI BOY PRO)
Definition
Electrical device allowing to nebulize a bronchodilator so that it can be administered as an inhalant. It delivers the medicine to the patient in a liquid phase going through the respiratory tract by creating small particles in the air to treat respiratory diseases.
The Pari Boy PRO is replacing the Pari Boy SX and PariMobile S which are no longer produced. Most of the accessories and spare-parts are compatible between the three models. if it is not the case the models are mentioned in the labels of the parts.
Specifications
Nebulizer functioning on the ambient air that generates aerosolised medication (finely dispersed airborne droplets) for inhalation by a patient with respiratory disorders.
Quality standards
Components
- 1 compressor with 1 nebulizer LC Sprint, including:
- 1 tubing (EEMDNECA206)
- 1 mouthpiece (EEMDNECA207)
- 1 power cable
- 1 filter (EEMDNECC601)
- 1 transport case
- 4 Nebulizer LC SPRINT 23G1001 reus.(EEMDNECA201) with 4 single use tubings (EEMDNECA206) and 4 reusable mouthpieces (EEMDNECA207)
- 5 reusable adult masks ref. 41G0740 (EEMDNECA202)
- 5 reusable child mask ref. 41G0741 (EEMDNECA203)
- 5 filters ref. 041G1002 (EEMDNECC601)
Technical specifications
- Portable nebulizer operated by an electrical compressor, functioning on air
- The flow, nebulization rate and inhalation time are preadjusted.
- Average functioning flow : 10.9 l/min
- Functioning pressure : 1.5 bar
- Filling volume is between 2 and 8 ml
- Sound level: +/- 54 dB (A)
- Power supply, mains: 220-240V 50Hz 0.7A 80W
- Protection against electric shocks
- Type BF
- Nebulizers, masks and mouthpieces autoclavable at 121°C or 134°C
- The tubing between nebulizer and compressor is single use and must be replaced (EEMDNECA206)
- CAUTION : if you nebulize medicines to newborn with T1 and child with T2 masks (not included in the basic composition), please ensure that the curved piece is present between the nebuliseur and the mask to enable the newborn/child to breath out.
Dimensions
- dimension 19.2 x 14.7 x 15 cm
- weight: 1.7 kg
To be Ordered Separately
Keep a sufficient stock of accessories and consumables:
- masks
- mouthpieces
- tubings, etc.
Instructions for use
- It is used in the treatment of acute severe asthma in adults and children
- It should also be used when a patient with moderate asthma exacerbations does not respond to treatment with an aerosol.
- It is used with special solutions for nebulizations, which are nebulized during 5 to 10 minutes.
- It can also be used with sodium chloride 6% for sputum induction necessary for the diagnosis of tuberculosis.
- For the treatment of baby and child, nebulizing must be realised under the surveillance of a healthcare worker
Maintenance
- Air filter removal (EEMDNECC601): after 200 operating hours
- Usual cleaning
- Sterilisation of the masks, mouthpieces
- autoclave at 121°C: conform to the recommendations of the IPC referent
- ONLY with the mask stabiliser accessory EEMDNECA211/ EEMDNECA212
- Never immerse the compressor in water or clean the compressor in running water
(Cf Introduction: Disinfection and sterilization in the field)
MSF requirements
Electrical device allowing to nebulize a medication to treat a respiratory illness in children and adults. Examples of medications that can be used with a nebulizer include: salbutamol, ipratropium and normal saline.


![[KMEDMHIE21-] (mod ICU) EXAMINATION-RESUSCITATION EQUIPMENT](/web/image/product.template/574340/image_256/%5BKMEDMHIE21-%5D%20%28mod%20ICU%29%20EXAMINATION-RESUSCITATION%20EQUIPMENT?unique=f72dda8)
![[KMEDMNUTI35] (module nut. inpatient) RESUSCITATION ITEMS 2021](/web/image/product.template/575291/image_256/%5BKMEDMNUTI35%5D%20%28module%20nut.%20inpatient%29%20RESUSCITATION%20ITEMS%202021?unique=dcc4b7f)
![[KMEDMHEE321A] (mod emergency) COMPLEMENTARY RESUSCITATION EQUIPMENT 2021](/web/image/product.template/574367/image_256/%5BKMEDMHEE321A%5D%20%28mod%20emergency%29%20COMPLEMENTARY%20RESUSCITATION%20EQUIPMENT%202021?unique=2272542)
![[KMEDMHWE221] (mod ward) COMPLEMENTARY RESUSCITATION EQUIPMENT](/web/image/product.template/572869/image_256/%5BKMEDMHWE221%5D%20%28mod%20ward%29%20COMPLEMENTARY%20RESUSCITATION%20EQUIPMENT?unique=d7fb3e5)
![[DORAIPRA2N-] IPRATROPIUM bromide, 0.25mg/ml, 1ml, sol. for nebulizer](/web/image/product.template/571337/image_256/%5BDORAIPRA2N-%5D%20IPRATROPIUM%20bromide%2C%200.25mg-ml%2C%201ml%2C%20sol.%20for%20nebulizer?unique=cb65670)
![[DORAIPRA5N-] IPRATROPIUM bromide, 0.25mg/ml, 2ml, sol. for nebulizer](/web/image/product.template/569470/image_256/%5BDORAIPRA5N-%5D%20IPRATROPIUM%20bromide%2C%200.25mg-ml%2C%202ml%2C%20sol.%20for%20nebulizer?unique=7ec9e27)
![[DORASALB2N2] SALBUTAMOL, 2mg/ml, 2.5ml, sol. for nebulizer, monodose](/web/image/product.template/573287/image_256/%5BDORASALB2N2%5D%20SALBUTAMOL%2C%202mg-ml%2C%202.5ml%2C%20sol.%20for%20nebulizer%2C%20monodose?unique=14b5e15)
![[EEMDNECA201] (nebulizer Pari) NEBULIZER LC SPRINT, reusable 23G1001](/web/image/product.template/571215/image_256/%5BEEMDNECA201%5D%20%28nebulizer%20Pari%29%20NEBULIZER%20LC%20SPRINT%2C%20reusable%2023G1001?unique=69e2bf5)
![[EEMDNECA202] (nebulizer Pari) ADULT MASK, reusable 41G0740](/web/image/product.template/571218/image_256/%5BEEMDNECA202%5D%20%28nebulizer%20Pari%29%20ADULT%20MASK%2C%20reusable%2041G0740?unique=4cdcffe)
![[EEMDNECA203] (nebulizer Pari) CHILD MASK, reusable 41G0741](/web/image/product.template/571219/image_256/%5BEEMDNECA203%5D%20%28nebulizer%20Pari%29%20CHILD%20MASK%2C%20reusable%2041G0741?unique=4cdcffe)
![[EEMDNECA206] (nebulizer Pari) CONNECTION TUBING, 1m20, 041G4591](/web/image/product.template/571160/image_256/%5BEEMDNECA206%5D%20%28nebulizer%20Pari%29%20CONNECTION%20TUBING%2C%201m20%2C%20041G4591?unique=d0832da)
![[EEMDNECA207] (nebulizer Pari) MOUTHPIECE, autoclavable 022G3050](/web/image/product.template/571155/image_256/%5BEEMDNECA207%5D%20%28nebulizer%20Pari%29%20MOUTHPIECE%2C%20autoclavable%20022G3050?unique=ca8d720)
![[EEMDNECA209] (nebulizer Pari) INFANT MASK T2 + bend (1-4 years) 41G0702](/web/image/product.template/569999/image_256/%5BEEMDNECA209%5D%20%28nebulizer%20Pari%29%20INFANT%20MASK%20T2%20%2B%20bend%20%281-4%20years%29%2041G0702?unique=7fca2be)
![[EEMDNECA210] (nebulizer Pari) INFANT MASK T1 + bend (0-1 years) 41G0701](/web/image/product.template/569997/image_256/%5BEEMDNECA210%5D%20%28nebulizer%20Pari%29%20INFANT%20MASK%20T1%20%2B%20bend%20%280-1%20years%29%2041G0701?unique=eec1ddc)
![[EEMDNECA211] (nebulizer Pari) STABILIZER for child mask 041G0748](/web/image/product.template/570813/image_256/%5BEEMDNECA211%5D%20%28nebulizer%20Pari%29%20STABILIZER%20for%20child%20mask%20041G0748?unique=554a040)
![[EEMDNECA212] (nebulizer Pari) STABILIZER for adult mask 41G0749](/web/image/product.template/570876/image_256/%5BEEMDNECA212%5D%20%28nebulizer%20Pari%29%20STABILIZER%20for%20adult%20mask%2041G0749?unique=064c0c3)
![[EEMDNECC601] (nebulizer Pari Boy Pro) AIR FILTER, 1 piece, 41G1002](/web/image/product.template/577820/image_256/%5BEEMDNECC601%5D%20%28nebulizer%20Pari%20Boy%20Pro%29%20AIR%20FILTER%2C%201%20piece%2C%2041G1002?unique=8e419ed)
![[SSCOSODC6V-] SODIUM chloride 6%, for nebulizer, 4 ml, vial](/web/image/product.template/572936/image_256/%5BSSCOSODC6V-%5D%20SODIUM%20chloride%206%25%2C%20for%20nebulizer%2C%204%20ml%2C%20vial?unique=92200da)
![[EEMDNECS601] (neb.PARI BOY PRO) FOOT BUMPER 130S1019](/web/image/product.template/577811/image_256/%5BEEMDNECS601%5D%20%28neb.PARI%20BOY%20PRO%29%20FOOT%20BUMPER%20130S1019?unique=40eebc5)
![[EEMDNECS602] (neb.PARI BOY PRO) SCREW HOUSING 041S9845](/web/image/product.template/577812/image_256/%5BEEMDNECS602%5D%20%28neb.PARI%20BOY%20PRO%29%20SCREW%20HOUSING%20041S9845?unique=8e419ed)
![[EEMDNECS603] (neb.PARI BOY PRO) COMPRESSOR assemb.EU 1.6bar-50Hz 130S1100](/web/image/product.template/577813/image_256/%5BEEMDNECS603%5D%20%28neb.PARI%20BOY%20PRO%29%20COMPRESSOR%20assemb.EU%201.6bar-50Hz%20130S1100?unique=40eebc5)
![[EEMDNECS604] (neb.PARI BOY PRO) MAINS CABLE+ WIRING HARNESS EU 130S1080](/web/image/product.template/577814/image_256/%5BEEMDNECS604%5D%20%28neb.PARI%20BOY%20PRO%29%20MAINS%20CABLE%2B%20WIRING%20HARNESS%20EU%20130S1080?unique=e7503b9)
![[EEMDNECS605] (neb.PARI BOY PRO) MAINS CABLE+ WIRING HARNESS UK 130S1083](/web/image/product.template/577815/image_256/%5BEEMDNECS605%5D%20%28neb.PARI%20BOY%20PRO%29%20MAINS%20CABLE%2B%20WIRING%20HARNESS%20UK%20130S1083?unique=e7503b9)
![[EEMDNECS606] (neb.PARI BOY PRO) PISTON ASSEMBLED 130S1224](/web/image/product.template/577816/image_256/%5BEEMDNECS606%5D%20%28neb.PARI%20BOY%20PRO%29%20PISTON%20ASSEMBLED%20130S1224?unique=98b6c85)
![[EEMDNECS607] (neb.PARI BOY PRO) MEMORY PISTON SEAL 130S1015](/web/image/product.template/577817/image_256/%5BEEMDNECS607%5D%20%28neb.PARI%20BOY%20PRO%29%20MEMORY%20PISTON%20SEAL%20130S1015?unique=8e419ed)
![[EEMDNECS608] (neb.PARI BOY PRO) COMPRESSOR FAN 130S1012](/web/image/product.template/577818/image_256/%5BEEMDNECS608%5D%20%28neb.PARI%20BOY%20PRO%29%20COMPRESSOR%20FAN%20130S1012?unique=e7503b9)
![[EEMDNECS609] (neb.PARI BOY PRO) CONNECTION PLATE with tubing 130S1341](/web/image/product.template/577819/image_256/%5BEEMDNECS609%5D%20%28neb.PARI%20BOY%20PRO%29%20CONNECTION%20PLATE%20with%20tubing%20130S1341?unique=554a040)