DEFIBRILLATOR Manual (Beneheart D3) + Accessories, English
Outdated Article
DEFIBRILLATEUR Manuel (Beneheart D3)
This article is discontinued. Spare parts and service until 31 March 2034.
Definition
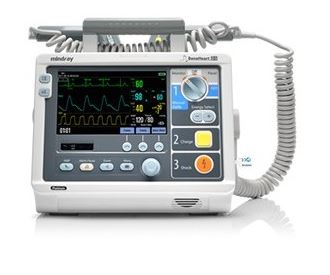
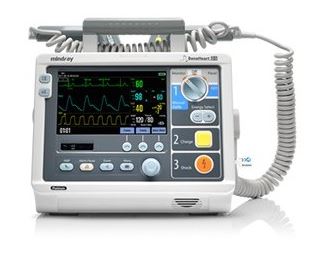
The Beneheart D3 is a portable defibrillator with four modes of operation: Monitor, Manual Defib, AED and Stimulus.
In Monitor mode, the unit is designed to monitor, display, view, record and print various physiological parameters and waveforms, including ECG, pulse oximetry (SpO2) and pulse rate (PR).
In AED mode, the unit automatically analyzes the patient's ECG rhythm and indicates whether or not a shockable rhythm is detected.
In Manual Defib Mode, the operator analyzes the patient's ECG. Defibrillation can be performed using paddles or MFE Pads. In Manual Defib Mode, you can also perform synchronised cardioversion.
The Stimulus Mode allows you to perform non-invasive transcutaneous pacing therapy.
Specifications
The equipment has the following main functions:
- Manual Defib Mode
- AED Mode
- Pacer Mode
- Monitor Mode
Quality standards
Components
- 1x) Li-ion battery
- (1x) 50 mm recorder
- (1x) External paddles Adu/ped
- (1x) tubing + adulte cuff (24-35 cm) for NIBP
- (1x) conductive gel
- (2x) Thermal paper (50mm*20m), set of 3 rolls
- (1x) MASIMO SpO2 extension cable
- (1x) LNCS DCI adult sensor for MASIMO SpO2
- (1x) 5 Leads-ECG trunk cable + wires, clip + electrodes
Technical specifications
- Mains power supply:
- mains voltage: 100 to 240 VAC (±10%)
- current: 1.8 to 0.8 A
- frequency: 50-60 Hz (±3 Hz)
- DC power supply (with DC/AC adapter):
- input voltage: 12 Vdc.
- Power consumption: 190 W
- Battery: 14.8 V / 3 Ah, intelligent lithium-ion battery, rechargeable and maintenance-free - a battery can be configured.
- Charging time:
- Less than 2 hours at 80% and less than 3 hours at 100% of with the appliance switched off
- Less than 3.5 hours at 80% and less than 4.5 hours at 100% when the power is on
- Defibrillation mode: manual defibrillation, synchronised cardioversion, ECD
- Defibrillation trace: biphasic truncated exponential biphasic (ETB) trace, self-compensating according to the patient's impedance.
- Defibrillation electrodes: set of external paddles supplied with pediatric paddles included, multifunction electrodes.
- External defibrillation: 20 to 200 Ω.
- Operating conditions:
- temperature: 0° to 45°C.
- humidity: 10-95% non-condensing
- altitude: -381 m to +4575 m
Dimensions
- (LxPxH): 288 × 203 × 275 mm
- Weight of main unit: 4.7kg
- Battery pack weight: 0.5kg
- Weight external pallet set: 0,8kg
Transport Dangerous Goods
Lithium-Ion battery
To be Ordered Separately
MFE (multi-function electrode) pads must be ordered separately
Instructions for use
Read the manual carefully before use
Precautions for Use
The device must be used in hospital settings with critical care units by qualified medical personnel who are trained in the operation of the device and who have received training in first aid, cardiac intensive care or defibrillation.
Maintenance
The equipment must be cleaned regularly. If the equipment operates in a particularly polluted or dusty environment, the cleaning frequency must be increased.
Recommended cleaning agents :
- mild soap (diluted)
- ammonia (diluted)
- bleach (sodium hypochlorite) (diluted)
- Hydrogen peroxide (hydrogen peroxide, 3%)
- Ethanol (70%)
- MSF Ref : Surfanios
Observe the following rules when cleaning the equipment :
- Switch off the unit, unplug the power cord and other cables, and remove the batteries.
- Clean the screen with a clean, soft cloth dampened with glass cleaner.
- Clean the outer surface of the machine with a clean soft cloth dampened with a window cleaner.
- Clean the pallet holder with a clean, soft cloth dampened with glass cleaner.
- Then wipe off the cleaning solution with a dry cloth if necessary.
- Allow the equipment to dry in a cool, ventilated area.
Storage
Storage temperature: -30° to 70°C
Storage humidity: 10-95%, non-condensing
Storage altitude: -381 m to +4575 m
Classification EC Regulation N° 1272/2008
Type of protection against electric shock: Class I
Degree of protection against harmful penetration of liquids:
- IPX4 in battery operation
- IPX1 in mains operation
MSF requirements
Defibrillator for synchronized cardioversion for supraventicular tachycardia, unstable atrial fibrillation, ventricular tachycardia, for defbrillation of ventricular fibrillation and pulsesless ventricular tachycardia.
This device is NST and designed to be used primarily in manual mode, and thus requires skilled personnel to perform rhythm analysis, energy level determination and use of specific modes : pacing, cardioversion.
The device should not be confused with AED which are the primary STD device aimed for CPR in MSF
Description approved by referent of OCB (Jonathan Delchambre) in October 2023.

![[EEMDDEFA201] (defib Beneheart D3) EXTERNAL PADDLES, Adu/ped 0651-30-77114](/web/image/product.template/582041/image_256/%5BEEMDDEFA201%5D%20%28defib%20Beneheart%20D3%29%20EXTERNAL%20PADDLES%2C%20Adu-ped%200651-30-77114?unique=5c65eeb)
![[EEMDDEFA202] (defib Beneheart D3) ECG WIRE 5-lead, adu/ped, 0.6m EL6502A](/web/image/product.template/581882/image_256/%5BEEMDDEFA202%5D%20%28defib%20Beneheart%20D3%29%20ECG%20WIRE%205-lead%2C%20adu-ped%2C%200.6m%20EL6502A?unique=f040248)
![[EEMDDEFA203] (defib Beneheart D3) SpO2 syst Masimo EXTENSION CABLE 562A](/web/image/product.template/581889/image_256/%5BEEMDDEFA203%5D%20%28defib%20Beneheart%20D3%29%20%20SpO2%20syst%20Masimo%20EXTENSION%20CABLE%20562A?unique=f040248)
![[EEMDDEFA204] (defib Beneheart D3) SPO2 SENSOR ped/inf., reusable U603-01P](/web/image/product.template/581883/image_256/%5BEEMDDEFA204%5D%20%28defib%20Beneheart%20D3%29%20SPO2%20SENSOR%20ped-inf.%2C%20reusable%20U603-01P?unique=f040248)
![[EEMDDEFA205] (Defib beneheart D3) NIBP TUBING +connectors 3m 62003009688](/web/image/product.template/581890/image_256/%5BEEMDDEFA205%5D%20%28Defib%20beneheart%20D3%29%20NIBP%20TUBING%20%2Bconnectors%203m%2062003009688?unique=f040248)
![[EEMDDEFA206] (Defib beneheart D3/D6) CUFF adult 25-35cm reusable CM1203](/web/image/product.template/581892/image_256/%5BEEMDDEFA206%5D%20%28Defib%20beneheart%20D3-D6%29%20%20CUFF%20adult%2025-35cm%20reusable%20CM1203?unique=f040248)
![[EEMDDEFA207] (defib Beneheart D3) ECG WIRE 5-lead, adu/ped 1-1.4m EL6504A](/web/image/product.template/581880/image_256/%5BEEMDDEFA207%5D%20%28defib%20Beneheart%20D3%29%20ECG%20WIRE%205-lead%2C%20adu-ped%201-1.4m%20EL6504A?unique=f040248)
![[EEMDDEFA208] (defib beneheart D3/D6) PADS CABLE reusable MR6702](/web/image/product.template/581886/image_256/%5BEEMDDEFA208%5D%20%28defib%20beneheart%20D3-D6%29%20PADS%20CABLE%20reusable%20MR6702?unique=f040248)
![[EEMDDEFA401] (defib Beneheart D3/D6) PAEDIATRIC CUFF, reusable CM1202](/web/image/product.template/581897/image_256/%5BEEMDDEFA401%5D%20%28defib%20Beneheart%20D3-D6%29%20%20PAEDIATRIC%20CUFF%2C%20reusable%20CM1202?unique=f040248)
![[EEMDDEFC211] (defib Beneheart D3) PAPER ROLL for printer 50mm*20m](/web/image/product.template/581891/image_256/%5BEEMDDEFC211%5D%20%28defib%20Beneheart%20D3%29%20PAPER%20ROLL%20for%20printer%2050mm%2A20m?unique=f040248)
![[EEMDDEFC212] (defib beneheart D3) CONDUCTIVE 250g GEL 15-25](/web/image/product.template/581881/image_256/%5BEEMDDEFC212%5D%20%28defib%20beneheart%20D3%29%20CONDUCTIVE%20250g%20GEL%2015-25?unique=f040248)
![[EEMDDEFC213] (defib Beneheart D3/D6) ELECTRODE PADS adult s.p. MR60](/web/image/product.template/581884/image_256/%5BEEMDDEFC213%5D%20%28defib%20Beneheart%20D3-D6%29%20ELECTRODE%20PADS%20adult%20s.p.%20MR60?unique=f040248)
![[EEMDDEFC214] (defib Beneheart D3/D6) ELECTRODE PADS ped. s.p. MR61](/web/image/product.template/581885/image_256/%5BEEMDDEFC214%5D%20%28defib%20Beneheart%20D3-D6%29%20ELECTRODE%20PADS%20ped.%20s.p.%20MR61?unique=f040248)
![[EEMDDEFS210] (defib Beneheart D3) BATTERY, Li-ion 14.8V, 3000mAh](/web/image/product.template/581888/image_256/%5BEEMDDEFS210%5D%20%28defib%20Beneheart%20D3%29%20BATTERY%2C%20Li-ion%2014.8V%2C%203000mAh?unique=f040248)