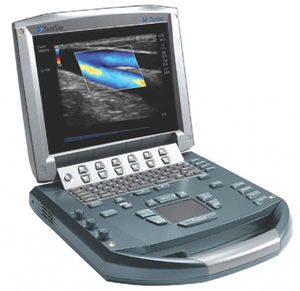
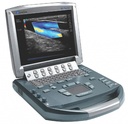
ULTRASOUND (Sonosite M-Turbo) + TRANSDUCER C60x
Discontinued Article
Documents
ULTRASOUND (Sonosite M-Turbo)
Stopped in 2023 because this model is no longer produced. It is replaced by the Edge 2 from Sonosite (see "replaced by" at bottom of the page)
the End of support for the M-Turbo
- April 30, 2025 Non-essential accessories (eg carry case)
- April 30 2028 Systems and transducers
Definition
Ultrasound-based diagnostic imaging apparatus used to visualize subcutaneous body structures. High image quality for abdominal, nerve, vascular, cardiac, venous access, pelvic and superficial imaging.
Specifications
Quality standards
Technical specifications
ULTRASOUND SYSTEM
- All-digital broadband architecture
- User interface and programmable controls:
- Softkeys to drive advanced features
- Programmable A and B keys
- Alphanumerical elastomeric QWERTY keyboard
- Track pad with select key for easy operation and navigation
- Doppler controls: angle, steer, scale, baseline, gain and volume
- Image acquisition keys: review, report, Clip Store, DVD, save
- Dynamic range: up to 165 dB
- Gray Scale: 256 shades
- Dedicated Auto Gain and examination keys to allow quick activation
- Display: LCD, 10.4" (26.4 cm), NTSC or PAL
- Power supply:
- Mains or battery-operated
- Lithium-ion battery: rechargeable <100Wh
- AC: universal power adapter, 100-240 VAC, 50/60 Hz input, 15 VDC output
- Weight: 3.04 kg
IMAGING MODES
- 2D / Tissue Harmonic Imaging / M-Mode
- Velocity Colour Doppler / Colour Power Doppler
- PW, PW Tissue Doppler and CW
- Doppler angle, correct after freeze
IMAGING PROCESSING
- SonoADAPT Tissue Optimisation
- SonoHD Imaging Technology
- Advanced Needle Visualisation
- Dual Imaging, Duplex Imaging, 2x pan/zoom, Dynamic range and gain
TRANSDUCER
- Broadband and Multifrequency: linear array, curved array, phased array, multiplane TEE and micro-convex
- Single Frequency: cardiac static pencil
CONNECTIVITY
- S-video (in/out) to VCR or DVD for record and playback
- RGB or DVI output to external LCD display
- Composite video output (NTSC/PAL) to VCR or DVD, video printer or external LCD display
- Audio output
- Integrated speakers
- EtherNet or wireless image/data transfer
- USB ports
- RS-232 transfer
ONBOARD IMAGE AND CLIP REVIEW
- 8GB internal flash memory storage capability (approx. 30 000 images or 960 2-second clips)
- Clip Store capability (maximum single clip length: 60 seconds)
- Cine review up to 255 frame-by-frame images
EXTERNAL DATA MANAGEMENT AND WIRELESS
- PC/Mac export configuration
- Export MPEG-4 (H.264), BMP, JPEG or HTML formats to a USB device
- DICOM image management
Dimensions
30.2 cm L x 27.4 cm W x 7.9 cm H
Transport Dangerous Goods
- UN3481 Lithium ion batteries contained in equipment
- Class: 9
- Packing Instructions: 967 Section II
- The label must include the UN code of the battery + an emergency number to contact in case of an incident
- Limit per package:
- Pax A/C (Passengers & Cargo Aircraft) = 5 kg
- CAO (Cargo Aircraft Only) = 5 kg
Supplied with the Article
- Battery (2 hours scanning time)
- International power cord
- Mini-dock (additional connection ports e.g. projector)
- Curved array transducer C60x/5-2 MHz curved array transducerbiopsy compatible
- USB memory drive 2 GB
- Bottle of acoustic gel
- Carry case
- User manual and service guides (several languages)
- 5-year warranty
To be Ordered Separately
Having the correct transducer(s) available is CRUCIAL for performing Ultrasound examinations.
Additional transducers are most likely required. See M-Turbo Ultrasound Probes (accessories).
Transducer needs vary by project site. To order the most suitable transducers for your site, please contact POCUS@msf.org or the POCUS advisor for further advice.
Consider ordering a backup of the transducer the project uses most frequently.
Recommended additional orders
MSF code | Item | Notes |
EDIMULSS206 | Charger L09823+ Power Cord - EU P00864 | 2-prong “EU” plug – Recommend 1 backup depending on plug type |
EDIMULSS207 | Charger L09823+ Power Cord - UK P07444 | 3-prong “UK” plug – Recommend 1 backup depending on plug type |
EDIMULSS801 | Battery - ultrasound | Recommend 1 backup |
EDIMULSC1CA* | Ultrasound Gel, 250 ml bot.) | Consumption: 1 bottle = 10 exams, order min 40/month |
SDISMDQA1T5** | Disinfection DETERGENT/DISINFECTANT for med. equip. 5 L tin + dosing pump | Diluted solution needed for daily IPC |
PELEVOLL207T*** | Voltage Protection - VOLTAGE LIMITER with timer, 180-265V, 7A, EUR, for fridge | For voltage stabilization, if needed |
*Ultrasound exams are not possible without gel! Please order months in advance, and recommend ordering more than necessary until exact usage/month is well known. This gel has a long shelf-life with no temperature requirements. NEVER use alcohol or oil based products (Sterilium, povidone-iodine, alcohol gel, gel cleansers) as these may damage the transducer. Sterile lubrication or plain WATER may be used as alternatives.
**Only 0.5% Anios Clean diluted solution, made fresh daily, should be used to clean transducers. This is required after EVERY patient exam. Surfanios is NOT approved as a cleaning solution and may damage the transducers. NEVER use alcohol or oil based products (Sterilium, povidone-iodine, alcohol gel, gel cleansers) as these may damage the transducer.
*** Only necessary if the electrical network is NOT already protected by MSF standard double-conversion UPS
Instructions for use
If gel is not readily available, it can be replaced with water. Never use alcohol, oil or any other fluids as they can damage the transducer.
If a patient shows infectious lesions, the end of the transducer must be covered with a male condom or glove during the examination.
For advanced obstetric applications, a vaginal transducer (ICT x/ 8-5MHz) can be ordered separately (see accessories). This transducer is intended for trained and experienced users. It must always be used with a male condom.
Precautions for Use
It is mandatory to protect the device with a double conversion UPS, and a voltage limiter with timer (see related articles below)
Maintenance
The transducer is not autoclavable.
After each examination, it must be cleaned and disinfected with a 0.5% detergent-disinfectant solution for medical equipment (either 25 ml = 1 stroke of dosing pump for 5 liters of water).
Storage
- Temperature between +10°C and +40°C
- Relative humidity: 15 - 95 %
- Atmospheric pressure: 700 - 1050 hPa
MSF requirements
This high-resolution ultrasound system offers such performance features as larger cart-based systems in a hand-carried, stand-alone unit.
5-year warranty on ultrasound system and transducers.


![[ADAPLAPA0LK] LOCK (Kensington) for laptop + key](/web/image/product.template/546634/image_256/%5BADAPLAPA0LK%5D%20LOCK%20%28Kensington%29%20for%20laptop%20%2B%20key?unique=bf9e276)
![[PELEVOLL207T] VOLTAGE LIMITER with timer (Voltguard) 180-265V, 7A, EUR](/web/image/product.template/557900/image_256/%5BPELEVOLL207T%5D%20VOLTAGE%20LIMITER%20with%20timer%20%28Voltguard%29%20180-265V%2C%207A%2C%20EUR?unique=44f7b6f)
![[SMSUCOND1--] CONDOM, lubricated + RESERVOIR, s.u.](/web/image/product.template/568812/image_256/%5BSMSUCOND1--%5D%20CONDOM%2C%20lubricated%20%2B%20RESERVOIR%2C%20s.u.?unique=0a37dd5)
![[EDIMULSA207] (ultrasound Micromaxx/M-Turbo/Edge 2) TROLLEY, generic](/web/image/product.template/573557/image_256/%5BEDIMULSA207%5D%20%28ultrasound%20Micromaxx-M-Turbo-Edge%202%29%20TROLLEY%2C%20generic?unique=937acd4)
![[EDIMULSA208] (ultrasound Micromaxx/M-Turbo) TROLLEY, Sonosite L12163](/web/image/product.template/579916/image_256/%5BEDIMULSA208%5D%20%28ultrasound%20Micromaxx-M-Turbo%29%20TROLLEY%2C%20Sonosite%20L12163?unique=d19c638)
![[EDIMULSA406] (ultrasound M-Turbo) ABDOMINAL TRANSDUCER C60x](/web/image/product.template/570116/image_256/%5BEDIMULSA406%5D%20%28ultrasound%20M-Turbo%29%20ABDOMINAL%20TRANSDUCER%20C60x?unique=478a81f)
![[EDIMULSA407] (ultrasound M-Turbo/Edge 2) VAGINAL TRANSDUCER ICTx](/web/image/product.template/570108/image_256/%5BEDIMULSA407%5D%20%28ultrasound%20M-Turbo-Edge%202%29%20VAGINAL%20TRANSDUCER%20ICTx?unique=514f368)
![[EDIMULSA408] (ultrasound M-Turbo) TRANSDUCER SUPERFICIAL STRUCT. HFL38x](/web/image/product.template/570110/image_256/%5BEDIMULSA408%5D%20%28ultrasound%20M-Turbo%29%20TRANSDUCER%20SUPERFICIAL%20STRUCT.%20HFL38x?unique=478a81f)
![[EDIMULSA409] (ultrasound M-Turbo) CARDIAC TRANSDUCER P21x](/web/image/product.template/570105/image_256/%5BEDIMULSA409%5D%20%28ultrasound%20M-Turbo%29%20CARDIAC%20TRANSDUCER%20P21x?unique=478a81f)
![[EDIMULSA410] (M-Turbo/Edge 2) PAED.ABDOMINAL(CURVELINEAR) TRANSDUCER C11x](/web/image/product.template/571245/image_256/%5BEDIMULSA410%5D%20%28M-Turbo-Edge%202%29%20PAED.ABDOMINAL%28CURVELINEAR%29%20TRANSDUCER%20C11x?unique=b4e0849)
![[EDIMULSA411] (US M-Turbo/Edge 2) PAEDIATRIC LINEAR TRANSDUCER L25X](/web/image/product.template/570889/image_256/%5BEDIMULSA411%5D%20%28US%20M-Turbo-Edge%202%29%20PAEDIATRIC%20LINEAR%20TRANSDUCER%20L25X?unique=44f7b6f)
![[EDIMULSA412] (ultrasound M-Turbo/Edge 2) NEONATAL TRANSDUCER P10x](/web/image/product.template/570986/image_256/%5BEDIMULSA412%5D%20%28ultrasound%20M-Turbo-Edge%202%29%20NEONATAL%20TRANSDUCER%20P10x?unique=e430896)
![[EDIMULSA413] (ultrasound M-Turbo) MINI DOCK L10401](/web/image/product.template/572182/image_256/%5BEDIMULSA413%5D%20%28ultrasound%20M-Turbo%29%20MINI%20DOCK%20L10401?unique=f928ae9)
![[EDIMULSA414] (ultrasound M-Turbo/Edge 2) CARRY CASE, P03673](/web/image/product.template/571334/image_256/%5BEDIMULSA414%5D%20%28ultrasound%20M-Turbo-Edge%202%29%20CARRY%20CASE%2C%20P03673?unique=9488726)
![[EDIMULSA415] (ultrasound M-Turbo/Edge 2) TRIPLE TRANSDUCER CONNECT P16535](/web/image/product.template/577846/image_256/%5BEDIMULSA415%5D%20%28ultrasound%20M-Turbo-Edge%202%29%20TRIPLE%20TRANSDUCER%20CONNECT%20P16535?unique=6ef6ac5)
![[EDIMULSC003] (ultrasound) COUPLING GEL, sterile, 20ml, sachet](/web/image/product.template/571681/image_256/%5BEDIMULSC003%5D%20%28ultrasound%29%20COUPLING%20GEL%2C%20sterile%2C%2020ml%2C%20sachet?unique=e26fce1)
![[EDIMULSC1CA] (Ultrasound) COUPLING AGENT, 250 ml bot.](/web/image/product.template/570107/image_256/%5BEDIMULSC1CA%5D%20%28Ultrasound%29%20COUPLING%20AGENT%2C%20250%20ml%20bot.?unique=514f368)
![[EDIMULSC411] (ultrasound) COVER + GEL for transducer,14x61cm,sterile,s.u.](/web/image/product.template/573082/image_256/%5BEDIMULSC411%5D%20%28ultrasound%29%20COVER%20%2B%20GEL%20for%20transducer%2C14x61cm%2Csterile%2Cs.u.?unique=ceb8cb2)
![[EDIMULSC412] (ultrasound) COVER + GEL transducer, 14x120cm, ster., s.u.](/web/image/product.template/574472/image_256/%5BEDIMULSC412%5D%20%28ultrasound%29%20COVER%20%2B%20GEL%20transducer%2C%2014x120cm%2C%20ster.%2C%20s.u.?unique=d19c638)
![[EDIMULSE8--] ULTRASOUND (Sonosite Edge 2) + TRANSDUCER rC60xi, armored c.](/web/image/product.template/578426/image_256/%5BEDIMULSE8--%5D%20ULTRASOUND%20%28Sonosite%20Edge%202%29%20%2B%20TRANSDUCER%20rC60xi%2C%20armored%20c.?unique=ebe9aac)
![[EDIMULSS206] (US Titan/Micromaxx/M-Turbo/Edge 2) POWER SUPPLY + CORD EU](/web/image/product.template/574918/image_256/%5BEDIMULSS206%5D%20%28US%20Titan-Micromaxx-M-Turbo-Edge%202%29%20POWER%20SUPPLY%20%2B%20CORD%20EU?unique=6e79fd8)
![[EDIMULSS207] (US Titan/Micromaxx/M-Turbo/Edge 2) POWER SUPPLY + CORD UK](/web/image/product.template/574919/image_256/%5BEDIMULSS207%5D%20%28US%20Titan-Micromaxx-M-Turbo-Edge%202%29%20POWER%20SUPPLY%20%2B%20CORD%20UK?unique=6e79fd8)
![[EDIMULSS401] (ultrasound Micromaxx/M-Turbo) BATTERY P07168](/web/image/product.template/570147/image_256/%5BEDIMULSS401%5D%20%28ultrasound%20Micromaxx-M-Turbo%29%20BATTERY%20P07168?unique=b95c5da)
![[EDIMULSS403] (ultrasound M-Turbo) TRANSDUCER NEST FRAME ASSY P07750](/web/image/product.template/579195/image_256/%5BEDIMULSS403%5D%20%28ultrasound%20M-Turbo%29%20TRANSDUCER%20NEST%20FRAME%20ASSY%20P07750?unique=72fb6d6)