(HemoCue Hb 301) CONTROL SOLUTION, kit 3 x 2 bottles
Valid Article
(HemoCue Hb 301) CONTROL
CAUTION
It is necessary to use the 3 levels of control (low, normal and high) when performing the quality controls
Definition
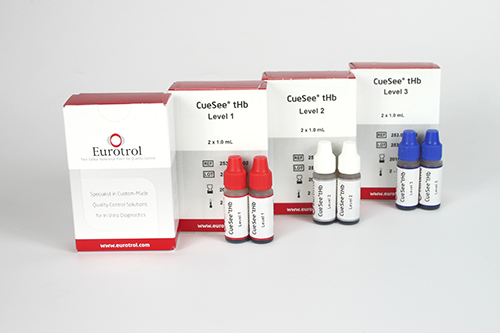
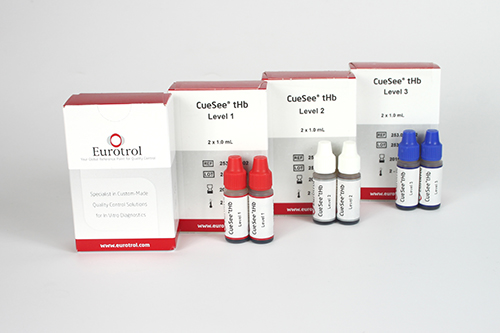
Control solutions (3 levels) for Hemocue Hb 301
Specifications
Clear red liquid in plastic dropper bottle
Technical specifications
Kit of 3 control solutions (3 x 2 dropper vials of 1.0 ml)
- Control solution, low
- Control solution, normal
- Control solution, high
The acceptable value range for each control solution is provided in the box.
Packaging & Labelling
Box with 2 x 1 ml vial for each control level
Instructions for use
Please consult the “Updated laboratory procedures, 2022” available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources.
https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Laboratory-Manual-page.aspx
For offline access, contact your laboratory advisor.
Precautions for Use
The controls for Hemocue Hb201+ cannot be used for the Hemocue 301 and vice-versa.
Storage
- Keep refrigerated between 2 and 8ºC
- Do not freeze
- Shelf life: 25 months
- Guaranteed minimum remaining shelf life at delivery: 1/3 of total shelf life
- Shelf life after opening: 30 days between 2 and 30°C

![[KMEDMHLA11B] (mod hospital lab) HEMOCUE cold chain](/web/image/product.template/572568/image_256/%5BKMEDMHLA11B%5D%20%28mod%20hospital%20lab%29%20HEMOCUE%20cold%20chain?unique=630812b)
![[KMEDMLAB16B] (laboratory module) BACT, HAEM, STOOLS, URINE EQ cold chain](/web/image/product.template/573180/image_256/%5BKMEDMLAB16B%5D%20%28laboratory%20module%29%20BACT%2C%20HAEM%2C%20STOOLS%2C%20URINE%20EQ%20cold%20chain?unique=06d3229)
![[KMEDMNUT34B] (module nutrition) DIAGNOSTIC MATERIALS cold chain 2021](/web/image/product.template/575290/image_256/%5BKMEDMNUT34B%5D%20%28module%20nutrition%29%20DIAGNOSTIC%20MATERIALS%20cold%20chain%202021?unique=3d64350)
![[KMEDMTRA01B] MODULE, TRANSFUSION, 50, part 2, cold chain](/web/image/product.template/569077/image_256/%5BKMEDMTRA01B%5D%20MODULE%2C%20TRANSFUSION%2C%2050%2C%20part%202%2C%20cold%20chain?unique=74751a4)
![[KMEDMTRA03B] MODULE, TRANSFUSION, children, part 2, cold chain](/web/image/product.template/569933/image_256/%5BKMEDMTRA03B%5D%20MODULE%2C%20TRANSFUSION%2C%20children%2C%20part%202%2C%20cold%20chain?unique=357758e)
![[ELAEHAEE3--] HAEMOGLOBIN PHOTOMETER (HemoCue Hb 301) tropicalized](/web/image/product.template/572148/image_256/%5BELAEHAEE3--%5D%20HAEMOGLOBIN%20PHOTOMETER%20%28HemoCue%20Hb%20301%29%20tropicalized?unique=1bb5108)