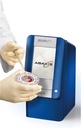
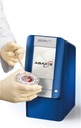
CLINICAL CHEMISTRY ANALYSER (Piccolo Xpress), 230V, 50-60Hz
Valid Article
CLINICAL CHEMISTRY ANALYSER (Piccolo)
Please check with your OC lab referent to ensure the appropriate choice of biochemistry equipment, depending of your context
Definition
Piccolo Xpress is a portable clinical chemistry system that can perform real time analysis of whole blood, serum or plasma samples, in self contained, single-use reagent discs.
It is used for routine multi-chemistry and electrolyte tests. It has an integrated intelligent Quality Control (iQC) system.
Specifications
Point of care analyzer performing blood chemistry tests that range from liver, kidney and metabolic functions to lipids and electrolytes.
Technical specifications
- Analyser hardware
- 10 digit numeric colour touch screen
- Built in thermal printer (external printer /LIS/EMR capable)
- Analyser software comprised of two matched programmes
- Sample size: 100 µl
- Sample type: Lithium heparin whole blood, serum or plasma
- Do not use EDTA as anticoagulant !
- Test time: approx 12 minutes
- Built-in intelligent Quality Control (iQC) system
- Mode of operation: continous
- Memory capacity: up to 5000 patient and quality control results
- Power requirements:
- 100 - 240 volts AC, 50 -60Hz
- or 16 volts DC, 5.0A
- Fluctuations supply voltage not to exceed +/- 10% of the nominal voltage
- Operating conditions
- Temperature range: between 15 and 25ºC. Warning: By MSF experience, the instrument does not work above 25°C even if the manufacturer states 32°C!
- Reaction temperature: 37ºC
- Relative humidity: 8 to 80%, non condensing
Dimensions
- Weight:
- analyser: 5.3 kg
- power adapter: 1.0 kg
- Height x Width x Depth: 325 x 149 x 210 mm
Supplied with the Article
Accessory kit supplied:
- Pipette fixed volume 100 µl (Minipette)
- Disposable minipette tips (96 tips/rack)
- Operators' manual ref. 1100-7009
- Power cord ref. 981-0090
- Power supply ref. 1988-0009
- Spare filter
- Adhesive paper roll ref. A1100-4410
To be Ordered Separately
- Test panels/cartridges and control solutions
- Carying case
- Adhesive paper roll
- Thermal paper
(see related items)
Instructions for use
Read the user manual.
Please consult the “Updated laboratory procedures, 2022” available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources.
https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Laboratory-Manual-page.aspx
For offline access, contact your laboratory advisor.
Precautions for Use
Secure all electrical devices in the laboratory with a UPS double conversion (see related articles below). To define the best sizing of the UPS, contact your biomedical or energy referent.



![[ELABPIAF0100] PIPETTE, AUTOMATIC, fixed volume 100 µl (Eppendorf)](/web/image/product.template/570353/image_256/%5BELABPIAF0100%5D%20PIPETTE%2C%20AUTOMATIC%2C%20fixed%20volume%20100%20%C2%B5l%20%28Eppendorf%29?unique=66f75fd)
![[ELABPIATY--] (aut.pip.) TIP YELLOW, 2-200µl (Eppdf)](/web/image/product.template/571110/image_256/%5BELABPIATY--%5D%20%28aut.pip.%29%20TIP%20YELLOW%2C%202-200%C2%B5l%20%28Eppdf%29?unique=66f75fd)
![[ELABPIDF100] PIPETTE, fixed volume (MiniPet), 100 µl blue](/web/image/product.template/572188/image_256/%5BELABPIDF100%5D%20PIPETTE%2C%20fixed%20volume%20%28MiniPet%29%2C%20100%20%C2%B5l%20%20blue?unique=578d3d7)
![[STSSBSVT2HL] (blds.syst.) TUBE, VACUUM, plastic, Li-HEPARIN, 2 ml green](/web/image/product.template/570347/image_256/%5BSTSSBSVT2HL%5D%20%28blds.syst.%29%20TUBE%2C%20VACUUM%2C%20plastic%2C%20%20Li-HEPARIN%2C%202%20ml%20green?unique=a3b54b2)
![[STSSBSVT5HL] (blds.syst.) TUBE, VACUUM, plastic, Li-HEPARIN, 4 ml green](/web/image/product.template/570523/image_256/%5BSTSSBSVT5HL%5D%20%28blds.syst.%29%20TUBE%2C%20VACUUM%2C%20plastic%2C%20%20Li-HEPARIN%2C%204%20ml%20green?unique=dd5b096)
![[ELAECCHA422] (clinical chem. Piccolo) CARRYING CASE, 1100-9011E](/web/image/product.template/573095/image_256/%5BELAECCHA422%5D%20%28clinical%20chem.%20Piccolo%29%20CARRYING%20CASE%2C%201100-9011E?unique=a9e1038)
![[ELAECCHC415] (Piccolo Xpress) THERMAL PAPER, adhesive, 1 roll 1100-4410](/web/image/product.template/572190/image_256/%5BELAECCHC415%5D%20%28Piccolo%20Xpress%29%20THERMAL%20PAPER%2C%20adhesive%2C%201%20roll%201100-4410?unique=49d2a38)
![[ELAECCHT401] (clinical chem. Piccolo) AMLYTE 13 PANEL, 400-0041](/web/image/product.template/572208/image_256/%5BELAECCHT401%5D%20%28clinical%20chem.%20Piccolo%29%20AMLYTE%2013%20PANEL%2C%20400-0041?unique=23d782d)
![[ELAECCHT402] (clinical chem. Piccolo) ELECTROLYTE PANEL, 400-0022](/web/image/product.template/572206/image_256/%5BELAECCHT402%5D%20%28clinical%20chem.%20Piccolo%29%20ELECTROLYTE%20PANEL%2C%20400-0022?unique=f3d31e9)
![[ELAECCHT403] (clinical chem. Piccolo) METLAC 12 PANEL, 400-0037](/web/image/product.template/572205/image_256/%5BELAECCHT403%5D%20%28clinical%20chem.%20Piccolo%29%20METLAC%2012%20PANEL%2C%20400-0037?unique=23d782d)
![[ELAECCHT404] (clinical chem. Piccolo) METLYTE 8 PANEL, 400-0023](/web/image/product.template/572218/image_256/%5BELAECCHT404%5D%20%28clinical%20chem.%20Piccolo%29%20METLYTE%208%20PANEL%2C%20400-0023?unique=9baf5cd)
![[ELAECCHT411] (clin.chem.Piccolo) KIT 10 CONTROLS, level 1 low A1100-9131E](/web/image/product.template/572216/image_256/%5BELAECCHT411%5D%20%28clin.chem.Piccolo%29%20KIT%2010%20CONTROLS%2C%20level%201%20low%20A1100-9131E?unique=8eabcab)
![[ELAECCHT412] (clin.chem.Piccolo) KIT 10 CONTROLS,level 2 high A1100-9132E](/web/image/product.template/572215/image_256/%5BELAECCHT412%5D%20%28clin.chem.Piccolo%29%20KIT%2010%20CONTROLS%2Clevel%202%20high%20A1100-9132E?unique=31fa4f5)
![[STSSBSVT4S-] (blds.syst.) TUBE, VACUUM, plastic, SERUM, 4 ml, red](/web/image/product.template/570517/image_256/%5BSTSSBSVT4S-%5D%20%28blds.syst.%29%20TUBE%2C%20VACUUM%2C%20plastic%2C%20SERUM%2C%204%20ml%2C%20red?unique=dd5b096)