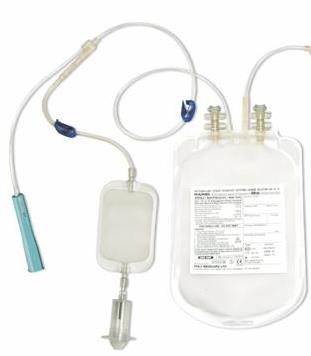
BLOOD BAG + diversion pouch, single, CPDA1, 250 ml, s.u.
Valid Article
BLOOD BAG, CPDA-1 + diversion pouch
Definition
An assembly of sterile devices intended to be used to collect whole blood from a donor. It includes a container (a single flexible bag) or five containers (pentabag) – one with anticoagulation solution, tubing, and an attached needle for venipuncture. It includes a pre-donation blood sampling device, and a needle stick prevention device.
After collection, the blood is tested, stored, and infused from the container when needed. The blood collected in the pentabag is, after testing, divided into the 4 satellite pouches.
Specifications
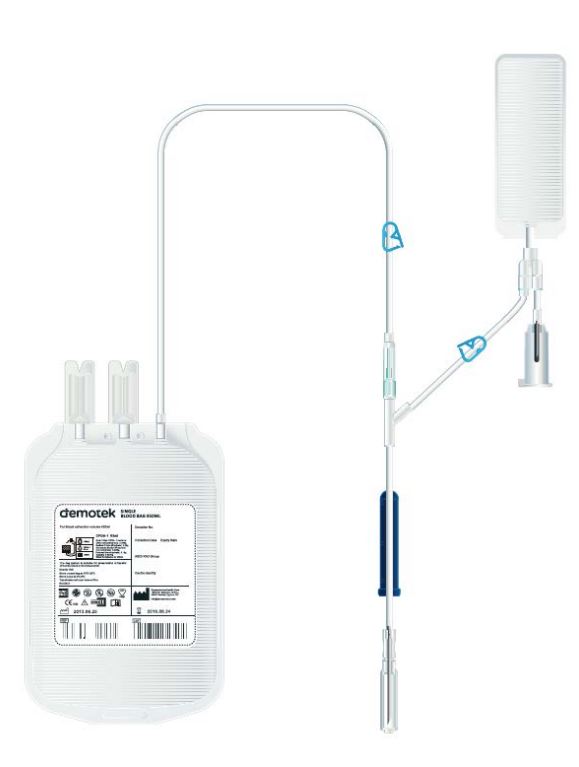
Schematic representation of the plastic (blood) container (according to ISO)
- 1: tamper evident protector(s)
- 2: transfer tube
- 3: means of closure (optional)
- 4: outlet port(s)
- 5: collection tube
- 6: tear line of protector
- 7: label area
- 8: tamper evident protective cap
- 9: blood-taking needle
- 10: needle hub
- 11: eyelets
- 12: puncturable non-resealable closure(s)
- 13: side slits
- a= Length ≥ 200 mm, internal diameter ≥ 2,7 mm, wall thickness ≥ 0,5 mm.
- b= Length ≥ 800 mm if used for gravitational collection, internal diameter ≥ 2,7 mm, wall thickness ≥ 0,5 mm.
- c= External view.
- D= Cross-sectional view.
add table below the graph
Quality standards
- ISO 3826-1, 2019, edition 3, +Amd 1 (2023) Plastics collapsible containers for human blood and blood components - Part 1: Conventional containers
- ISO 3826-2, 2008, edition 1, (confirmed 2022) Plastics collapsible containers for human blood and blood components — Part 2: Graphical symbols for use on labels and instruction leaflets
- ISO 3826-3, 2006, edition 1, (confirmed 2021) Plastics collapsible containers for human blood and blood components - Part 3: Blood bag systems with integrated feature
Quality Standards Comment
USP for CPDA-1 whole blood
Technical specifications
Sterile, for single use
BAG
- PVC or other polyolefins (in many countries, national pharmacopoeias specify formulations of different plastics materials)
- Transparent, almost colourless to allow visual inspection of the content
- Flexible
- With 2 outlet ports for the administration of blood through a transfusion set. The ports have a puncturable non-resealable closure port septum placed at 14 (+/- 2 mm) from the top of the port. Each outlet port is fitted with a hermetically sealed, tamper-evident protector (to maintain sterility of internal surface)
- Fitted with holes for suspension
TUBINGS
- Collection tube: the single bag (150 ml, 250 ml, 450 ml) and the parent pouch of the pentabag has a tubing connected to a needle for collecting blood. The collection tubing is fitted with a clamp, and an additional clamp for the diversion pouch
- Transfer tube: only for the pentabag: connects the parent 450 ml container to the different 100 ml satellite pouches. This tubing is fitted with a device which first acts like a seal and then, when opened, permits the free flow of blood. There is a clamp per tubing and pouch.
- Flexible but kink-resistant
- They can be sealed hermetically and do not collapse under normal use
- Under visual inspection, the tubing shall not display cracks, blisters, kinks or other defects
- Internal diameter minimum 2.7 mm
- Wall thickness: minimum 0.5 mm
- Minimum length: 80 cm for the collection tube, 20 cm for the transfer tubing.
BLOOD TAKING NEEDLE
- Stainless steel
- Integral with the tubing at the donor's end
- The needle has a visible or tactile means of indicating the position of the needle bevel
- Cap to protect the needle and ensure integrity (tamper-evident) and sterility of the system
- Needle guard, mounted on the tubing to prevent accidental needle-stick injury (it slides over the soiled needle after blood taking and is self-locking)
ANTICOAGULANT SOLUTION
CPDA-1 (anticoagulant citrate phosphate dextrose adenine solution). Chemical composition of the solution according to the pharmacopoeia.
Allows anticoagulation and preservation of blood (may be stored for up to 35 days at 1- 6°C)
Quantity of anticoagulant per pouch:
| Bag capacity | Anticoagulant (CPDA-1) |
| 150 ml | 21 ml |
| 250 ml | 35 ml |
| 450 ml | 63 ml |
Note: only the parent 450 pouch of the pentabag contains anticoagulant solution, the satellite pouches do not contain any liquid.
DIVERSION POUCH = pre-donation sampling device (PDS): device integrated in the donor line of blood bag systems and designed to separate the first volume (at least 35 ml) of donated blood, fitted with a tube holder for Vacutainer tube system. The PDS is integrated in the donor line through a Y-piece, such that blood may only flow into the pre-donation sampling device or into the blood bag.
Labelling
All labels must meet the requirements of the ISO 3826-1
Label on the pouch, printed information (if sufficient space)
- name + address of manufacturer (or responsible supplier)
- description of the content + intended use
- nature, composition and volume of anticoagulant and volume of blood to be collected
- statement about sterility and non-pyrogenicty
- product code and lot designation
- reference to the IFU
- for single use only
- do not use if visible sign of deterioration
- do not vent
Label on the pouch, requirements
- label does not cover the pouch completely: part remains visible for inspection
- enough space available to add user information: number of blood bags, date of collection, expiry date, ABO group + Rh, test results, volume
- no diffusion of print from the label, print remains legible at all times
- label does not separate from the pouch even if wet
Packaging & Labelling
The bag and tube are arranged in a sealed over-package in a manner which will minimise the tube from kinking and acquiring a permanent set.
Minimal info on the over-package label:
- manufacturer name+address, description of contents, product code & lot designation, expiry date
- instruction that the pouch shall not be used more than N days after removal from the over-package. (N is defined by the manufacturer, unless otherwise required e.g. by regulation, is usually 15 days)
Note: if a transparent over-package is used, this information has to appear on the label of the pouch, in case multiple pouches are in the same over-package, the expiry date (+ instruction limited use after opening) will appear only on the first visible pouch.
Label on the shipping box should be visible when paletted, minimal info:
- manufacturer name+address, description of contents, product code & lot designation, expiry date
- if the transit container functions as an over-package; instruction that the pouch shall not be used more than N days after removal from the over-package
Instructions for use
It is strongly recommended to order the transfusion module which contains all the necessary items to perform 50 transfusions (see related articles below).
Precautions for Use
Do not use if there is any sign of deterioration of the packaging.
Blood Bag containing whole blood should not come in contact with ice at any stage
A brown deposit in the tubing near the needle (caramelization of dextrose) indicates that the solvent has evaporated and the anticoagulant solution is not valid any more. In this case, blood bags must be discarded and you should inform your supply centre.
Storage
- Store below 25º C, Protect from sunlight
- Do not freeze
MSF requirements
The bloodbags with a diversion pouch (SINSBABS+++) have replaced the traditional bloodbags (SINSBABT+++) because of increased availability, reduction of bacterial risk and practical use for pre-transfusion testing.
Extra Tables
ISO 3826-1: dimensions of the bloodbag / dimensions des poches de sang (mm) | ||||
| Nominal capacity (ml) | inside width = w1 | inside height = h1 | size label area = w2+/-5 | size label area = h2 +/- 5 |
| 100 | 75 | 120 | 60 | 85 |
| 250 | 120 | 130 | 90 | 85 |
| 400 | 120 | 170 | 105 | 105 |

![[ELABFOBB1--] TUBE STRIPPER, for blood bag tubing, manual](/web/image/product.template/572385/image_256/%5BELABFOBB1--%5D%20TUBE%20STRIPPER%2C%20for%20blood%20bag%20tubing%2C%20manual?unique=c22cfc0)
![[ELAEBDRE2--] BLOOD COLLECTION MONITOR, 230V (Hemotek2)](/web/image/product.template/570165/image_256/%5BELAEBDRE2--%5D%20BLOOD%20COLLECTION%20MONITOR%2C%20230V%20%28Hemotek2%29?unique=792d8cb)
![[ELAESEAE1--] BLOOD BAG TUBE SEALER (Delcon HemoWeld T), 115-230V](/web/image/product.template/572964/image_256/%5BELAESEAE1--%5D%20BLOOD%20BAG%20TUBE%20SEALER%20%28Delcon%20HemoWeld%20T%29%2C%20115-230V?unique=792d8cb)
![[KMEDMTRA03A] MODULE, TRANSFUSION, children part 1](/web/image/product.template/569931/image_256/%5BKMEDMTRA03A%5D%20MODULE%2C%20TRANSFUSION%2C%20children%20part%201?unique=bf8002b)