HEPATITIS B TEST HBsAg, wb, 1 test (Bioline HBsAg 01FK10W)
Valid Article
HEPATITIS B TEST (Bioline HBsAg)
CAUTION
This test is worded and codified by unit to make the order easier, but it is always packaged in kit of 30.
Definition
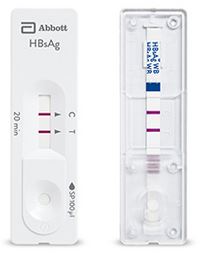
Rapid immunochromatographic test for the qualitative detection of hepatitis B surface antigen used for pretransfusional screening.
Specifications
Components
- 30 test devices
- instructions for use
Technical specifications
- Test device (30) single pouched
- One-step rapid test (± 20 minutes)
- Sample type: venous whole blood (not validated on capillary blood)
- Sample volume: 100 µl
- Analytical sensitivity on WHO International Biological reference preparation: 2.06IU/ml
- According to WHO Prequalification report (version August 2020)
- Sensitivity on serum: 100 % (95% CI: 98.1-100%)
- Specificity on serum: 99.0% (95% CI:97.2-99.8%)
- With internal control line
- Without positive and negative external controls
Packaging & Labelling
Kit of 30 tests
Instructions for use
Follow the instructions in the leaflet.
IMPORTANT: After several complaints of false positive faint lines testing plasma on the Bioline HBsAg WB test received from several MSF field projects, MSF validated its use on venous whole blood only, regardless of the IFU claiming that plasma can be used.
Sample type: 100µl venous whole blood (not validated on capillary blood)
If you are considering the use of this test for another indication than pretransfusional screening (i.e. screening of chronic hepatitis B infection), additional testing should be performed to determine if the patient should be considered for antiretroviral therapy (i.e. HBeAg, HBV VL). It is recommended to consult your respective OC laboratory advisor and medical director before use outside pretransfusional screening.
Please consult the “Updated laboratory procedures, 2022” available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources.
https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Laboratory-Manual-page.aspx
For offline access, contact your laboratory advisor.
- See: Blood transfusion, MSF, 2019
- See: Updated laboratory procedures, MSF, 2022
- See: WHO Model List of Essential In Vitro Diagnostics 2023
- See: HepatitisB Bioline SSDTHBTE30T SOP, MSF, 2022
- See: Bioline HBsAg WB SSDTHBTE30T BA, 2019
- See: Bioline HBsAg S&PL SSDTHBTE30T BA, MSF, 2022
- See: MSF diagnostic packages, MSF, 2023
Precautions for Use
All biological samples must be considered as potentially infectious and handled with the usual precautions (compulsary wearing of gloves, hand washing, etc.).
Storage
- Store between 1 and 40ºC
- Do not freeze
- Shelf life: 24 months
- Not to be used beyond expiry date
Waste management
Incinerate the tests and the used materials.
MSF requirements
Rapid test prequalified by WHO.
No need for cold chain.







![[ELABPIAA0200] PIPETTE, AUTOMATIC, adjustable vol. 20-200 µl (Eppendorf)](/web/image/product.template/574431/image_256/%5BELABPIAA0200%5D%20PIPETTE%2C%20AUTOMATIC%2C%20adjustable%20vol.%2020-200%20%C2%B5l%20%28Eppendorf%29?unique=02388ef)
![[ELABPIATY--] (aut.pip.) TIP YELLOW, 2-200µl (Eppdf)](/web/image/product.template/571110/image_256/%5BELABPIATY--%5D%20%28aut.pip.%29%20TIP%20YELLOW%2C%202-200%C2%B5l%20%28Eppdf%29?unique=0a70ae5)
![[ELABPIDF100] PIPETTE, fixed volume (MiniPet), 100 µl blue](/web/image/product.template/572188/image_256/%5BELABPIDF100%5D%20PIPETTE%2C%20fixed%20volume%20%28MiniPet%29%2C%20100%20%C2%B5l%20%20blue?unique=a708ea3)
![[STSSBSVT5E-] (blds.syst.) TUBE, VACUUM, plastic, K2EDTA, 4ml, purple](/web/image/product.template/570499/image_256/%5BSTSSBSVT5E-%5D%20%28blds.syst.%29%20TUBE%2C%20VACUUM%2C%20plastic%2C%20K2EDTA%2C%204ml%2C%20purple?unique=f03d256)
![[SSDTHBTE10T2] HEPATITIS B TEST HBsAg (Determine 2),ser/pl/wb,1 test 7D2943](/web/image/product.template/573731/image_256/%5BSSDTHBTE10T2%5D%20HEPATITIS%20B%20TEST%20HBsAg%20%28Determine%202%29%2Cser-pl-wb%2C1%20test%207D2943?unique=7f234ee)