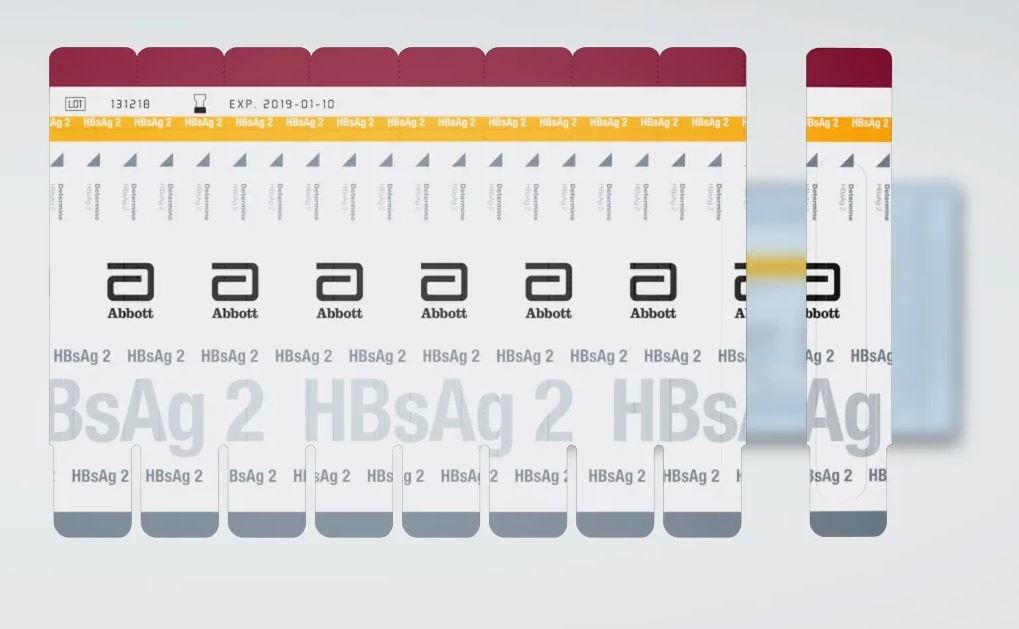
HEPATITIS B TEST HBsAg (Determine 2),ser/pl/wb,1 test 7D2943
Valid Article
HEPATITIS B TEST HBsAg (Determine 2)
CAUTION
This test is worded and codified by unit to make the order easier, but it is always packaged in kit of 100.
Definition
Rapid immunochromatographic test for the qualitative detection of hepatitis B surface antigen.
Used for the screening of blood donors and patients for hepatitis B detection.
Specifications
Technical specifications
- Reagent strips attached by 10 (stripable)
- One-step rapid test (± 20 minutes)
- Sample type: serum, plasma or whole blood (capillary or venous)
- Sample volume: 50µl
- According WHO prequalification report, January 2020:
- Sensitivity on serum: 100 % (98.2-100)
- Specificity on serum/plasma: 100% (98.8-100)
- With internal control line
- Without external positive and negative controls
Packaging & Labelling
Aluminium foil pack of 100 tests (= 10 cards), with zip closure
Transport Dangerous Goods
Transport not regulated
Instructions for use
Follow the instructions in the leaflet.
Provide an automatic pipette or disposable pipettes (MiniPets) and corresponding tips for the application of the 50 µl sample (see related articles below).
For serum or plasma samples (preferred sample types, see below):
- No additional reagent is required
- For plasma samples, use EDTA tubes
For whole blood samples:
- Validated on venous and capillary blood
- Chase buffer is mandatory, ref: 7D2243 or 7D2243R (see related articles)
- For venous samples, use EDTA tubes
- For capillary samples, use the specific EDTA capillary tubes, ref. 7D2222 or 7D2227 (see related articles)
If you are considering the use of this test for another indication than pretransfusional screening (i.e. screening of chronic hepatitis B infection), additional testing should be performed to determine if the patient should be considered for antiretroviral therapy (i.e. HBeAg, HBV VL). It is recommended to consult your respective OC laboratory advisor and medical director before use outside pretransfusional screening.
Please consult the “Updated laboratory procedures, 2022” available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources.
https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Laboratory-Manual-page.aspx
For offline access, contact your laboratory advisor.
- See: Blood transfusion, MSF, 2019
- See: Updated laboratory procedures, MSF, 2022
- See: WHO Model List of Essential In Vitro Diagnostics 2023
- See: HepatitisB Determine2 SSDTHBTE10T2 SOP, MSF, 2022
- See: Determine HBsAg 2 SSDTHBTE10T2 BA, MSF 2018
- See: MSF diagnostic packages, MSF, 2023
Precautions for Use
All biological samples must be considered as potentially infectious and handled with the usual precautions (compulsary wearing of gloves, hand washing, etc.).
Storage
- Store between 2 and 30ºC
- Do not freeze
- Shelf life: 18 months
- Guaranteed minimum remaining shelf life at delivery: 1/3 of the total shelf life
- Not to be used beyond expiry date
Waste management
Incinerate the tests and the used materials.
Classification EC Regulation N° 1272/2008
Not classified as hazardous





![[ELABPIAA0100] PIPETTE, AUTOMATIC, adjustable vol. 10-100 µl (Eppendorf)](/web/image/product.template/570956/image_256/%5BELABPIAA0100%5D%20PIPETTE%2C%20AUTOMATIC%2C%20adjustable%20vol.%2010-100%20%C2%B5l%20%28Eppendorf%29?unique=4283584)
![[ELABPIATY--] (aut.pip.) TIP YELLOW, 2-200µl (Eppdf)](/web/image/product.template/571110/image_256/%5BELABPIATY--%5D%20%28aut.pip.%29%20TIP%20YELLOW%2C%202-200%C2%B5l%20%28Eppdf%29?unique=0a70ae5)
![[ELABPIDF50-] PIPETTE, fixed volume (MiniPet), 50 µl yellow](/web/image/product.template/571134/image_256/%5BELABPIDF50-%5D%20PIPETTE%2C%20fixed%20volume%20%28MiniPet%29%2C%2050%20%C2%B5l%20yellow?unique=9e0a09d)
![[ELABTUCA1E-] (Determine rapid test) TUBE, CAPILLARY, EDTA 7D2222/7D2227](/web/image/product.template/571066/image_256/%5BELABTUCA1E-%5D%20%28Determine%20rapid%20test%29%20TUBE%2C%20CAPILLARY%2C%20EDTA%207D2222-7D2227?unique=348850d)
![[SSDTDETC101] (Determine rapid test) BUFFER CHASE, 2.5 ml, 7D2243/7D2243R](/web/image/product.template/570532/image_256/%5BSSDTDETC101%5D%20%28Determine%20rapid%20test%29%20BUFFER%20CHASE%2C%202.5%20ml%2C%207D2243-7D2243R?unique=3e1a193)
![[STSSLANCSAM2] SAFETY LANCET, medium flow, needle 21G x 1.8mm, green, s.u.](/web/image/product.template/569100/image_256/%5BSTSSLANCSAM2%5D%20SAFETY%20LANCET%2C%20medium%20flow%2C%20needle%2021G%20x%201.8mm%2C%20green%2C%20s.u.?unique=4e619c7)
![[SSDTHBTE30T] HEPATITIS B TEST HBsAg, wb, 1 test (Bioline HBsAg 01FK10W)](/web/image/product.template/568984/image_256/%5BSSDTHBTE30T%5D%20HEPATITIS%20B%20TEST%20HBsAg%2C%20wb%2C%201%20test%20%28Bioline%20HBsAg%2001FK10W%29?unique=0cc858e)
![[KMEDMTRA01A] MODULE, TRANSFUSION, 50, part 1](/web/image/product.template/569059/image_256/%5BKMEDMTRA01A%5D%20MODULE%2C%20TRANSFUSION%2C%2050%2C%20part%201?unique=a602693)
![[KMEDMTRA03A] MODULE, TRANSFUSION, children part 1](/web/image/product.template/569931/image_256/%5BKMEDMTRA03A%5D%20MODULE%2C%20TRANSFUSION%2C%20children%20part%201?unique=5c1c774)