BUDESONIDE 80mcg/FORMOTEROL 4.5 mcg/p, 120 puffs, aer.
Valid Article
BUDESONIDE / FORMOTEROL aerosol
- In the WHO list of Essential Medicines 2023 (Dry powder inhaler: 100 or 200 mcg + 6 mcg per dose)
Therapeutic Action
Combination of inhaled corticosteroid (ICS) and long-acting beta2-agonist (LABA) inhaled bronchodilator:
- Budesonide: a glucocorticoid with an anti-inflammatory action in the lungs, resulting in reduced symptoms and exacerbations of asthma with less adverse effects than with systemic corticosteroids.
- Formoterol: a selective beta2-adrenergic agonist that produces relaxation of bronchial smooth muscle in patients with reversible airways obstruction. The bronchodilation effect sets in rapidly, within 1-3 minutes after inhalation, and has a duration of 12 hours after a single dose.
Indications
Treatment of moderate to severe persistent asthma in children
Instructions for use
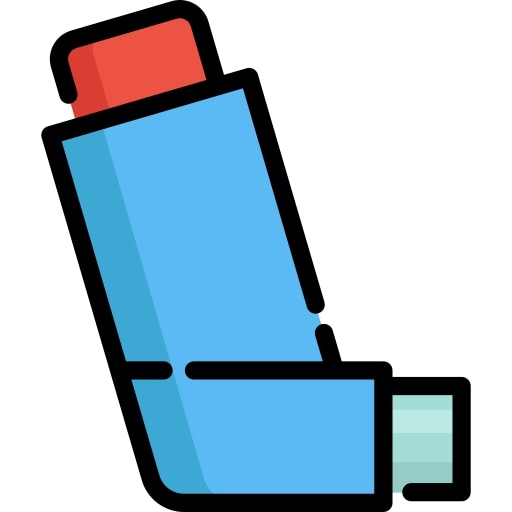
The combination of budesonide and formoterol is available as a suspension or solution (pressurised metered-dose inhaler) or as a dry powder (dry-powder inhaler) to inhale by mouth using a specially designed inhaler:
- Pressurised metered-dose inhaler (MDI): small canister containing a mixture of medicine and pressurised propellant. The pressure from the propellant delivers the medicine from the canister when the canister is pushed down into the boot/actuator.
- Dry-Powder Inhaler (DPI): breath-actuated device that delivers the medicine as a fine, dry powder contained in a capsule or blister that is punctured by the device before use.
Manufacturers may express the content per puff as a "metered dose" (excluding the valve, i.e the quantity of drug substance contained in the device metering chamber) or as a "delivered dose" (excluding the mouthpiece, i.e. the quantity of drug substance delivered to the user). Always consult the product leaflet.
The inhalers generally supplied by MSF are pressurised metered-dose inhalers. Refer to the information leaflet for appropriate instructions for use/inhalation according the inhaler model.
Pressurised metered-dose inhalers are CFC free presentation. Chlorofluorocarbons (CFCs) are harmful to the ozone layer and HFA (hydrofluoroalkane) propellant is a more effective, and environmentally-friendly alternative.
Precautions for Use
Do not use for the treatment of an acute asthma attack.
In children, use an inhalation chamber/spacer with or without a mask (depending on the age of the child) with every administration of pressurised metered-dose inhalers.
Do not use any mouthpiece other than the one supplied with the inhaler.
Transport Dangerous Goods
UN transport code:
- UN 1950 - Aerosols, non-flammable (each not exceeding 1 L capacity)
- Transport Class 2.2 - Non-flammable, non-toxic gases
Transport is only regulated for large quantities of aerosols.
Storage
- Below 25ºC
- Used pressurised aerosols: do not pierce or incinerate. Empty all residual gas, then bury.

![[SMSUSPAC4M-] SPACER, single patient, medium mask](/web/image/product.template/569846/image_256/%5BSMSUSPAC4M-%5D%20SPACER%2C%20single%20patient%2C%20medium%20mask?unique=b26010c)
![[SMSUSPAC4S-] SPACER, single patient, small mask](/web/image/product.template/569849/image_256/%5BSMSUSPAC4S-%5D%20SPACER%2C%20single%20patient%2C%20small%20mask?unique=4d85b8a)