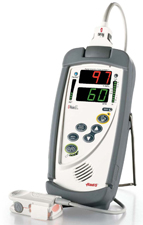
PULSE OXIMETER (Masimo RAD-5) + accessories
Valid Article
Documents
PULSE OXYMETER (Masimo RAD-5)
Definition
Portable device allowing to monitor arterial oxygen saturation (% SpO2) as well as the pulse rate (beats/minute). This non invasive continuous measurement is taken thanks to a sensor placed on a finger, a toe or an ear lobe.
Specifications
Quality standards
Components
- 1 x Pulse oxymeter RAD-5 ref. 9112 or 9214
- 1 x rubber protective case ref. 1981
- 1 x patient extension cable RED LNC-04
- 2 x reusable universal adult sensors LNCS-DCI ref. 1863
- 1 reusable paediatric multisite sensor LNCS-YI ref. 2258
- 2 boxes of 100 adhesive tapes (wraps) each for neonate sensor LNCS-YI ref.1598
- 1 box of 100 adhesive tapes (wraps) for sensor LNCS-DCI ref. 1597
- 4 batteries AA (non- rechargeable!)
- 1 x English and French instructions for use ref. 13261 - 13017
Technical specifications
- Case with LED screen displaying:
- pulse rate (bpm)
- oxygen saturation (SpO2)
- perfusion index (PI)
- warning lights (sensor condition, system failure and low battery)
- Saturation accuracy: 70-100%
- Pulse rate accuracy: 25-240 bpm
- Can be used continuously in adults, children, neonates
- Visual and audio alarms
- Runs on 4 batteries type R6
- Batteries capacity up to 48 hours
- Operating conditions
- temperature: 5ºC to 40ºC
- humidity: 5% to 95%, non condensing
- altitude: 1060 to 500 mbar
- Weight: ± 0.4 kg
- Dimensions: 17.5 x 7.6 x 3.6 cm
Instructions for use
Remarks
- The new sensors are compatible with the previous Nellcor models: N20PA/NPB40. The connection between the sensor and the cable is always grey (sensor) - white (cable).
- The new model of Masimo RAD-5 has a red connection and needs a red connection cable LNC-04 ref. 2055 for the sensors (wraps).
- The old models (<2010) with white connector need the white connection cable LNC-4 ref. 2017 for the sensors (wraps).
Be aware that multiple factors can affect the accuracy of a pulse oximeter reading, such as poor circulation, skin pigmentation, skin thickness, skin temperature, current tobacco use, and use of fingernail polish.
Make diagnosis and treatment decisions based on trends in pulse oximeter readings over time, rather than absolute thresholds.
Precautions for Use
To prevent batteries from deteriorating, it is recommended to remove them when the device is unused for a prolonged period or stored under damp conditions.
Storage
- Storage temperature: -40ºC to 70ºC
MSF requirements
Essential for any emergency room, ICU and delivery room.




![[KMEDKHCX3OP] OPD, COMPLEMENTARY PART observation beds, minimum package](/web/image/product.template/572652/image_256/%5BKMEDKHCX3OP%5D%20OPD%2C%20COMPLEMENTARY%20PART%20observation%20beds%2C%20minimum%20package?unique=f41629f)
![[KMEDMHCE23-] (mod OPD) MEDICAL EQUIPMENT 2021](/web/image/product.template/574347/image_256/%5BKMEDMHCE23-%5D%20%28mod%20OPD%29%20MEDICAL%20EQUIPMENT%202021?unique=f41629f)
![[KMEDKHCX3--] OPD, COMPLEMENTARY PART observation beds](/web/image/product.template/573068/image_256/%5BKMEDKHCX3--%5D%20OPD%2C%20COMPLEMENTARY%20PART%20observation%20beds?unique=e5feae7)
![[KMEDMHIE21-] (mod ICU) EXAMINATION-RESUSCITATION EQUIPMENT](/web/image/product.template/574340/image_256/%5BKMEDMHIE21-%5D%20%28mod%20ICU%29%20EXAMINATION-RESUSCITATION%20EQUIPMENT?unique=22350c3)
![[KMEDMHAE221] (mod AMP) COMPLEMENTARY RESUSCITATION EQUIPMENT](/web/image/product.template/572665/image_256/%5BKMEDMHAE221%5D%20%28mod%20AMP%29%20COMPLEMENTARY%20RESUSCITATION%20EQUIPMENT?unique=22350c3)
![[KMEDMHWE221] (mod ward) COMPLEMENTARY RESUSCITATION EQUIPMENT](/web/image/product.template/572869/image_256/%5BKMEDMHWE221%5D%20%28mod%20ward%29%20COMPLEMENTARY%20RESUSCITATION%20EQUIPMENT?unique=22350c3)
![[KMEDMHDE31-] (mod delivery & neonate) MEDICAL EQUIPMENT 2021](/web/image/product.template/574359/image_256/%5BKMEDMHDE31-%5D%20%28mod%20delivery%20%26%20neonate%29%20MEDICAL%20EQUIPMENT%202021?unique=de395b9)
![[KMEDMSUP04EQ] (supplementary unit 2018) MODULE EQUIPMENT](/web/image/product.template/572596/image_256/%5BKMEDMSUP04EQ%5D%20%28supplementary%20unit%202018%29%20MODULE%20EQUIPMENT?unique=62e6069)
![[KMEDMNUTI35] (module nut. inpatient) RESUSCITATION ITEMS 2021](/web/image/product.template/575291/image_256/%5BKMEDMNUTI35%5D%20%28module%20nut.%20inpatient%29%20RESUSCITATION%20ITEMS%202021?unique=f928ae9)
![[KMEDMHEE321A] (mod emergency) COMPLEMENTARY RESUSCITATION EQUIPMENT 2021](/web/image/product.template/574367/image_256/%5BKMEDMHEE321A%5D%20%28mod%20emergency%29%20COMPLEMENTARY%20RESUSCITATION%20EQUIPMENT%202021?unique=d19c638)
![[EEMDPOXA401] (oximeter Masimo) SENSOR adult, reus. LNCS DCI 1863](/web/image/product.template/571391/image_256/%5BEEMDPOXA401%5D%20%28oximeter%20Masimo%29%20SENSOR%20adult%2C%20reus.%20LNCS%20DCI%201863?unique=e11cd1e)
![[EEMDPOXA402] (oximeter Masimo) SENSOR multisite paed/neon LNCS YI 2258](/web/image/product.template/571387/image_256/%5BEEMDPOXA402%5D%20%28oximeter%20Masimo%29%20SENSOR%20multisite%20paed-neon%20LNCS%20YI%202258?unique=56d156f)
![[EEMDPOXA403] (oximeter Masimo) CABLE, extension <2010, white, LNC-4 2017](/web/image/product.template/571388/image_256/%5BEEMDPOXA403%5D%20%28oximeter%20Masimo%29%20CABLE%2C%20extension%20%3C2010%2C%20white%2C%20LNC-4%202017?unique=3f6516f)
![[EEMDPOXA406] (oximeter Masimo) CABLE, extension, red, LNC-04 2055](/web/image/product.template/571381/image_256/%5BEEMDPOXA406%5D%20%28oximeter%20Masimo%29%20CABLE%2C%20extension%2C%20red%2C%20LNC-04%202055?unique=56d156f)
![[EEMDPOXA407] (oximeter Masimo) RUBBER PROTECTION, orange 1982](/web/image/product.template/570689/image_256/%5BEEMDPOXA407%5D%20%28oximeter%20Masimo%29%20RUBBER%20PROTECTION%2C%20orange%201982?unique=36a8e00)
![[EEMDPOXA408] (oximeter Masimo) WRAPS reusable fr LNCS YI sensor, 1602](/web/image/product.template/569212/image_256/%5BEEMDPOXA408%5D%20%28oximeter%20Masimo%29%20WRAPS%20reusable%20fr%20LNCS%20YI%20sensor%2C%201602?unique=8ce41ad)
![[EEMDPOXA409] (oximeter RAD-5) USER MANUAL](/web/image/product.template/572276/image_256/%5BEEMDPOXA409%5D%20%28oximeter%20RAD-5%29%20USER%20MANUAL?unique=e26fce1)
![[EEMDPOXA410] (oximeter Masimo) RUBBER PROTECTION, red 1981](/web/image/product.template/572702/image_256/%5BEEMDPOXA410%5D%20%28oximeter%20Masimo%29%20RUBBER%20PROTECTION%2C%20red%201981?unique=b4e0849)
![[EEMDPOXA411] (oximeter Masimo) RUBBER PROTECTION, blue 2097](/web/image/product.template/572700/image_256/%5BEEMDPOXA411%5D%20%28oximeter%20Masimo%29%20RUBBER%20PROTECTION%2C%20blue%202097?unique=b4e0849)
![[EEMDPOXA412] (oximeter Masimo) RUBBER PROTECTION, grey 1842](/web/image/product.template/569704/image_256/%5BEEMDPOXA412%5D%20%28oximeter%20%20Masimo%29%20RUBBER%20PROTECTION%2C%20grey%201842?unique=44f7b6f)
![[EEMDPOXA413] (oximeter Masimo) SENSOR Pediatric, reus. LNCS-DC-IP](/web/image/product.template/571272/image_256/%5BEEMDPOXA413%5D%20%28oximeter%20Masimo%29%20SENSOR%20Pediatric%2C%20reus.%20LNCS-DC-IP?unique=9488726)
![[EEMDPOXC404] (oximeter Masimo) WRAP adhesive for LNCS sensor, 1597](/web/image/product.template/571379/image_256/%5BEEMDPOXC404%5D%20%28oximeter%20Masimo%29%20WRAP%20adhesive%20for%20LNCS%20sensor%2C%201597?unique=e11cd1e)
![[EEMDPOXC405] (oximeter Masimo) WRAP adhesive for LNCS sensor, 1598](/web/image/product.template/571401/image_256/%5BEEMDPOXC405%5D%20%28oximeter%20Masimo%29%20WRAP%20adhesive%20for%20LNCS%20sensor%2C%201598?unique=e11cd1e)
![[EEMDPOXC406] (oximeter Masimo) SPO2 SENSOR s.u. adult/neo LNCS Neo-3](/web/image/product.template/571456/image_256/%5BEEMDPOXC406%5D%20%28oximeter%20Masimo%29%20SPO2%20SENSOR%20s.u.%20adult-neo%20LNCS%20Neo-3?unique=9488726)
![[PELEBATTR06] BATTERY rechargeable (R6/AA) NiMH, 1.2V](/web/image/product.template/549284/image_256/%5BPELEBATTR06%5D%20BATTERY%20rechargeable%20%28R6-AA%29%20NiMH%2C%201.2V?unique=5093827)