ORTHOPEDIC TRACTION FRAME TRILLAT, inox, L 65 to 90 cm
STD
EHOETRAF1--
Valid Article
Account code:
60100
HS Code:
901890
Last Updated on:
22/11/2024, 22:15:23
Former
Code(s):
-X
L0908 - Orthopaedic and traumatological traction instruments, reusable
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
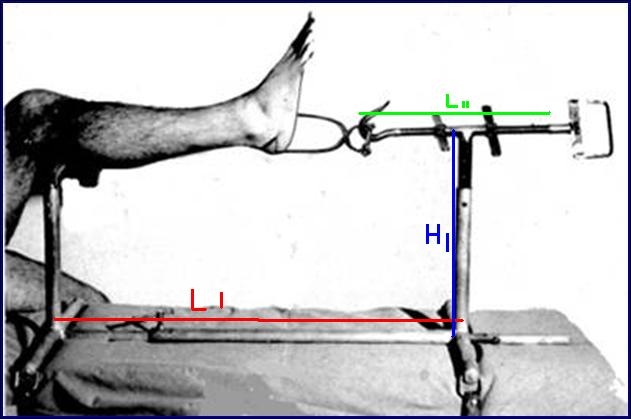
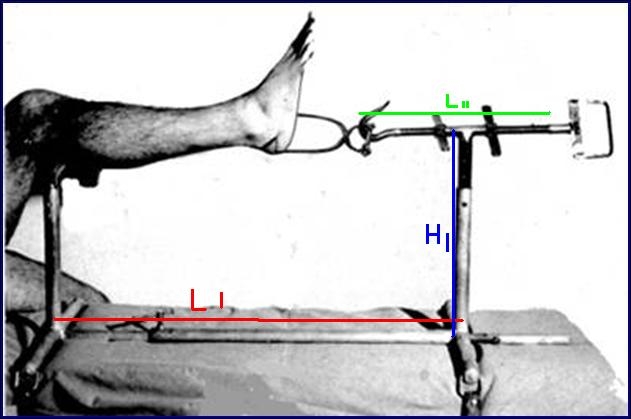
ORTHOPEDIC TRACTION FRAME TRILLAT
Definition
Stainless steel frame allowing the reduction of fractures of the tibia and fibula in the operation theatre by placing the limb under traction and making a cast.
Specifications
Technical specifications
- Material: austenitic stainless steel (SAE 304 or European norm 1.4301)
- Adjustable length from 65 to 90 cm
- Adjustable height from 20 to 40 cm
- Traction rod, adjustable in length by a screw system, fitted with a hook enabling the traction on the Boehler bow
- Leg-rest
- Non sterile
Packaging & Labelling
Unit packaging of the disassembled frame
Instructions for use
Some restricted information has been hidden. Sign in
to see this information
Maintenance
After use, clean and disinfect with a detergent/disinfectant solution for surfaces /non-invasive medical equipment. Prepare use-solution in concentration required. Always read the label and product information before use. Do not mix with other products. Make sure to wet the surfaces completely, use a wipe or towel, keep them wet for the whole exposure time.
MSF requirements
Optional item for the Operating Theatre Part, medical equipment for 1 operating room.
Some restricted information has been hidden. Sign in
to see this information

![[ESURBOWB09-] BOW, WIRE TRACTION, BOEHLER 9 x 16 cm + NAIL, 76-44-01](/web/image/product.template/570178/image_256/%5BESURBOWB09-%5D%20BOW%2C%20WIRE%20TRACTION%2C%20BOEHLER%209%20x%2016%20cm%20%2B%20NAIL%2C%2076-44-01?unique=911cb9b)
![[ESURBOWB11-] BOW, WIRE TRACTION, BOEHLER 11 x 21 cm + NAIL, 76-44-03](/web/image/product.template/570184/image_256/%5BESURBOWB11-%5D%20BOW%2C%20WIRE%20TRACTION%2C%20BOEHLER%2011%20x%2021%20cm%20%2B%20NAIL%2C%2076-44-03?unique=911cb9b)
![[ESURBOWB15-] BOW, WIRE TRACTION, BOEHLER 15 x 21 cm + NAIL, 76-44-05](/web/image/product.template/570182/image_256/%5BESURBOWB15-%5D%20BOW%2C%20WIRE%20TRACTION%2C%20BOEHLER%2015%20x%2021%20cm%20%2B%20NAIL%2C%2076-44-05?unique=911cb9b)
![[ESURNAIS15-] NAIL, STEINMANN, trocar point, 15 cm, Ø 4 mm 76-44-15](/web/image/product.template/569091/image_256/%5BESURNAIS15-%5D%20NAIL%2C%20STEINMANN%2C%20trocar%20point%2C%2015%20cm%2C%20%C3%98%204%20mm%2076-44-15?unique=85fb2c3)
![[ESURNAIS21-] NAIL, STEINMANN, trocar point, 21 cm, Ø 4 mm 76-44-21](/web/image/product.template/569094/image_256/%5BESURNAIS21-%5D%20NAIL%2C%20STEINMANN%2C%20trocar%20point%2C%2021%20cm%2C%20%C3%98%204%20mm%2076-44-21?unique=85fb2c3)
![[ESURNAIS26B] NAIL, STEINMANN, trocar point, 26 cm, Ø 4 mm 76-17-79](/web/image/product.template/569099/image_256/%5BESURNAIS26B%5D%20NAIL%2C%20STEINMANN%2C%20trocar%20point%2C%2026%20cm%2C%20%C3%98%204%20mm%2076-17-79?unique=85fb2c3)
![[KSURBTRN13-] TRACTION SET 13 instruments + 16 Nails of Steinmann](/web/image/product.template/570342/image_256/%5BKSURBTRN13-%5D%20TRACTION%20SET%2013%20instruments%20%2B%2016%20Nails%20of%20Steinmann?unique=55eac07)