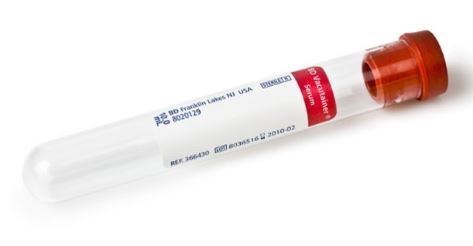
(blds.syst.) TUBE, VACUUM, plastic, SERUM, 2ml, red
Valid Article
(blood sampling) VACUUM TUBE
Definition
Vacuum sterile tube, with or without additive, intended to contain a blood specimen.
Specifications
Quality Standards Comment
EN ISO 6710 : 2017: Single-use containers for human venous blood specimen collection (ISO 6710:2017)
Material
- Tube: polyethylene tetraphtalate (PET) and polypropylene (PP) except STSSBSVTG5S: glass
- Cap: polymer (low density Polyethylene resin)
- Closure: bromobutyl elastomer
Technical specifications
- Vacuum tube, sterile, for single use
- Dimensions: 75 mm x 13 mm Ø
- Perforable plug
- Paper label
- All 3 brands compatible with the Vacutainer blood sampling system
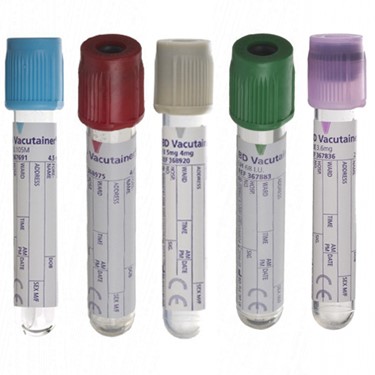
STSSBSVT2E-
- EDTA K2 tube, volume 5 ml, vacuum 2 ml
- EDTA K2 sprayed on the internal wall
- plug colour: purple
STSSBSVT5E-
- EDTA K2 tube, volume 5 ml, vacuum 4 ml
- EDTA K2 sprayed on the internal wall
- plug colour: purple
STSSBSVT2HL
- heparinized tube, volume 5 ml, vacuum 2 ml (except Lind-Vac: 3ml)
- contains : lithium heparin
- plug colour: green
STSSBSVT5HL
- heparinized tube, volume 5 ml, vacuum 4 ml
- contains : lithium heparin
- plug colour: green
STSSBSVT2S-
- siliconized serum tube, volume 5 ml, vacuum 2 ml
- plain tube, no additive (except Vacutainer, with clot activator (silicium))
- plug colour: red (brick)
STSSBSVT5S-
- siliconized serum tube, volume 5 ml, vacuum 4 ml
- plain tube, no additive (except Vacutainer, with clot activator (silicium))
- plug colour: red (brick)
STSSBSVTG5S
- glass
- serum tube, volume 5 ml
- plain tube, uncoated, no additives
- plug colour: red (brick)
STSSBSVT2C-
- citrate tube, volume 5 ml, vacuum 2.7 ml (except Vacuette: 3 ml)
- contains: buffered trisodium citrate solution
- plug colour: translucent light blue
Packaging & Labelling
Box of 100.
Instructions for use
EDTA tubes are aimed for haematological tests and blood transfusion tests.
Heparinized tubes are to be used for biochemistry tests (i-STAT) in sleeping sickness/trypanosomiasis projects for the mini Anion Exchange Centrifugation Technique (mAECT).
Serum tubes are aimed for screening tests and biochemistry tests.
Glass serum tubes are aimed for early diagnosis and monitoring of coagulation abnormalities based on a clotting test (e.g. snake bites)
Citrate tubes are aimed for coagulation studies.
2 ml tubes are to be used for newborns and children.
Please consult the “Updated laboratory procedures, 2022” available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources.
https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Laboratory-Manual-page.aspx
For offline access, contact your laboratory advisor.
Please consult the “Manual of nursing Care Procedures, MSF, 2020” available online via the Nursing care working Group sharepoint page.
Storage
- Store between 4 and 25°C, Protect from sunlight ‐ Protect from humidity
- Do not freeze
- Shelf life: depending on the manufacturer
- Not to be used beyond expiry date
Waste management
To be destroyed by incineration.
(Cf Public Health Engineering In Precarious situations, MSF, 2010, Chapter 6)
MSF requirements
Closed system ensuring a good security for the sample and the practitioner. Plastic tubes which can not break.

![[STSSBSVVH1-] (blds. syst.) HOLDER for VACUUM TUBE with needle ejector](/web/image/product.template/570528/image_256/%5BSTSSBSVVH1-%5D%20%28blds.%20syst.%29%20HOLDER%20for%20VACUUM%20TUBE%20with%20needle%20ejector?unique=aadad37)
![[STSSBSVVN21] (blds.syst.) NEEDLE, sterile, 21G (Vacutainer)](/web/image/product.template/570526/image_256/%5BSTSSBSVVN21%5D%20%28blds.syst.%29%20NEEDLE%2C%20sterile%2C%2021G%20%28Vacutainer%29?unique=040ec3f)
![[STSSBSVVN23W] (blds.syst.) SAMPLING SET, with wings, 23 G (Vacutainer)](/web/image/product.template/570527/image_256/%5BSTSSBSVVN23W%5D%20%28blds.syst.%29%20SAMPLING%20SET%2C%20with%20wings%2C%2023%20G%20%28Vacutainer%29?unique=040ec3f)