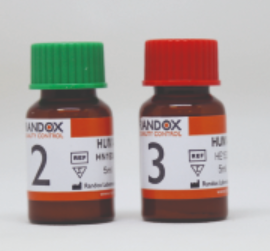
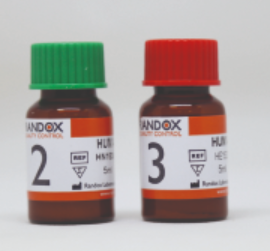
(clin.chem./electr.) CONTROL KIT, level 2+3, 2x5x5ml HS2611
STD
ELAECANT007
Valid Article
HS Code:
382290
Last Updated on:
04/10/2024, 14:44:04
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
(clininical chemistry/electrolytes) CONTROL KIT, level 2+3
Definition
Human multi-sera controls, level 2 and 3, to perform daily internal quality control on clinical chemistry analyzers (e.g. Spotchem EL and EZ)
Specifications
Some restricted information has been hidden. Sign in
to see this information
Technical specifications
- Lyophilized
Packaging & Labelling
2 x 5 x 5ml bottles
Instructions for use
Some restricted information has been hidden. Sign in
to see this information
Storage
- Unopened: Store between 2 and 8°C. Stable until expiry date.
- Reconstituted: Store between 2 and 8°C for 7 days, or between 15 and 25°C for 8 hours, or between -18 and -24°C for 28 days.
Waste management
Substance with biohazardous characteristics. Please contact your watsan referent for advice on proper disposal.
Detailed hazard and precautionary information can be found in the safety data sheet (SDS).
Biohazard, containing serum from human origin
MSF requirements
Use with Spotchem EZ/EL
Some restricted information has been hidden. Sign in
to see this information

![[ELAECCHE6--] CLINICAL CHEMISTRY ANALYSER (Spotchem EZ)](/web/image/product.template/581641/image_256/%5BELAECCHE6--%5D%20CLINICAL%20CHEMISTRY%20ANALYSER%20%28Spotchem%20EZ%29?unique=51760f0)
![[ELAEELAE4--] ELECTROLYTE ANALYSER (Spotchem EL SE-1520) 100-240V 50-60Hz](/web/image/product.template/568867/image_256/%5BELAEELAE4--%5D%20ELECTROLYTE%20ANALYSER%20%28Spotchem%20EL%20SE-1520%29%20100-240V%2050-60Hz?unique=0bd30c4)
![[ELAEELAT403] (an. Spotchem EL/EZ) MULTILEVEL CONTROL, 2x3x2ml 77708/6](/web/image/product.template/569149/image_256/%5BELAEELAT403%5D%20%28an.%20Spotchem%20EL-EZ%29%20MULTILEVEL%20CONTROL%2C%202x3x2ml%2077708-6?unique=60997c1)